正在加载图片...
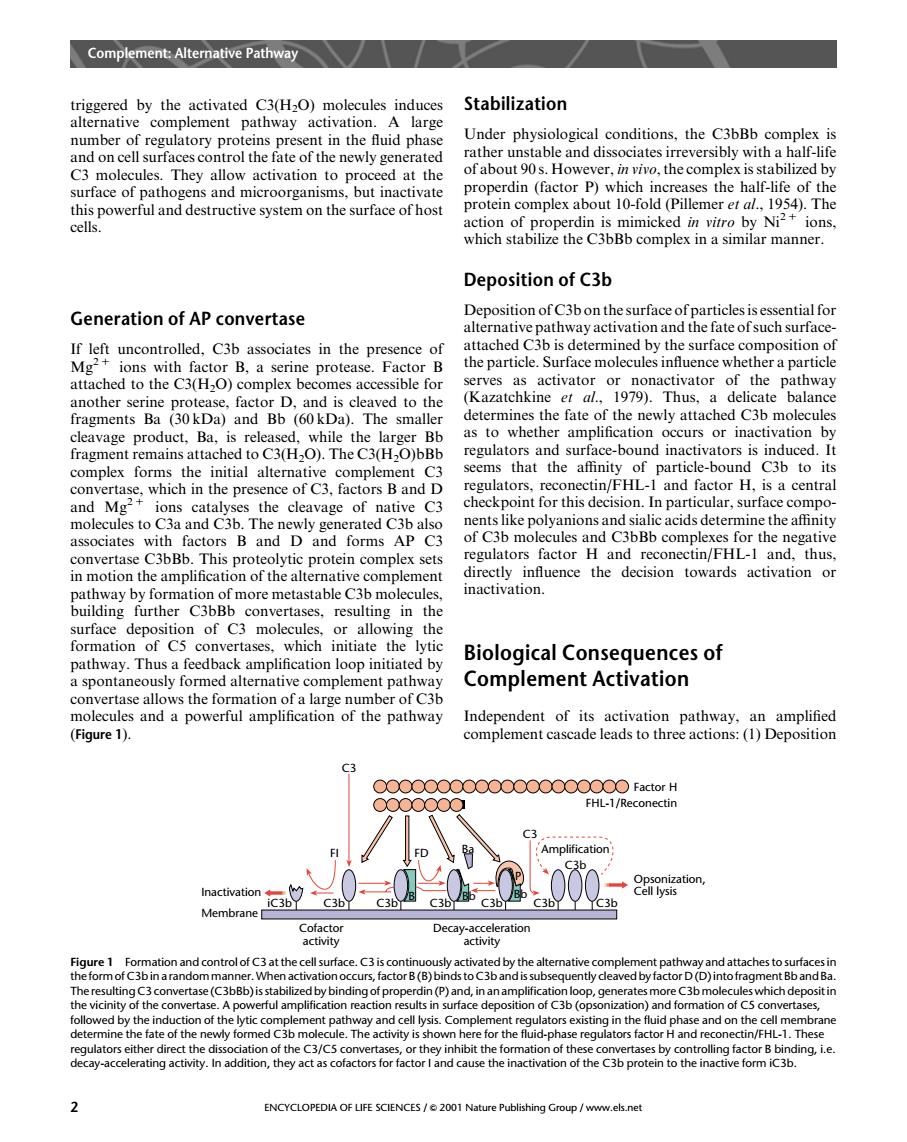
Complmen Ateative Pathway triggered by the activated C3(H2O)molecules induces Stabilization alternative complement pathway activation.A large r of regulatory proteins present in the fluid phase Under physiological conditions,the C3bBb complex is cel he newly gener rather unstable and dissociates irreversibly with a half-life surface of pathogens and microorganisms.but inactivate ofabout 9s.However.nthe this powerful and destructive system on the surface of host l.1954.The cells. of r erdin is mimicked in vitro by Ni?+ions which stabilize the C3bBb complex in a similar manner. Deposition of C3b Generation of AP convertase Deposition of c3bon the surface ofparticles is essential fo alternative pathway activation and the fate ofsuch surface ce er a partic attached to the C3(H-O)complex becomes accessible for (Kazatchkine)Thus. determines the fate of the newly attached C3b molecules th as to whether amplification occurs or inactivation by fragment remains attached to C3(H2O).The C3(H2O)bBb san sur 步一 tor H is a central con presence ctin/FHL-I and fad check point for this decision.In particular.surface compo les to Caand Cab The associates with factors B and D and forms AP C3 convertase C3bBb.This proteolytic prote in complex sets in on of t com inactivation. buildins further C3bBb in the surface depos tion of C3 molecules or allowing the formation of C5 convertases.which initiate the pathway.Thus Biological Consequences of feed d by ed alte mber of Car Complement Activation the fo tion molecules and a powerful amplification of the pathway Independent of its activation pathway,an amplified (Figure 1). complement cascade leads to three actions:(1)Deposition FHL-1/Reconectin C3 c36 Membrane h actor B(B) (D)int 91 rface depos .Th ENCYCLOPEDIA OF LIFE SCIENCES/001 Nature Publishing Group/www.els.nettriggered by the activated C3(H2O) molecules induces alternative complement pathway activation. A large number of regulatory proteins present in the fluid phase and on cell surfaces control the fate of the newly generated C3 molecules. They allow activation to proceed at the surface of pathogens and microorganisms, but inactivate this powerful and destructive system on the surface of host cells. Generation of AP convertase If left uncontrolled, C3b associates in the presence of Mg2 1 ions with factor B, a serine protease. Factor B attached to the C3(H2O) complex becomes accessible for another serine protease, factor D, and is cleaved to the fragments Ba (30 kDa) and Bb (60 kDa). The smaller cleavage product, Ba, is released, while the larger Bb fragment remains attached to C3(H2O). The C3(H2O)bBb complex forms the initial alternative complement C3 convertase, which in the presence of C3, factors B and D and Mg2 1 ions catalyses the cleavage of native C3 molecules to C3a and C3b. The newly generated C3b also associates with factors B and D and forms AP C3 convertase C3bBb. This proteolytic protein complex sets in motion the amplification of the alternative complement pathway by formation of more metastable C3b molecules, building further C3bBb convertases, resulting in the surface deposition of C3 molecules, or allowing the formation of C5 convertases, which initiate the lytic pathway. Thus a feedback amplification loop initiated by a spontaneously formed alternative complement pathway convertase allows the formation of a large number of C3b molecules and a powerful amplification of the pathway (Figure 1). Stabilization Under physiological conditions, the C3bBb complex is rather unstable and dissociates irreversibly with a half-life of about 90 s. However, in vivo, the complex is stabilized by properdin (factor P) which increases the half-life of the protein complex about 10-fold (Pillemer et al., 1954). The action of properdin is mimicked in vitro by Ni2 1 ions, which stabilize the C3bBb complex in a similar manner. Deposition of C3b Deposition of C3b on the surface of particles is essential for alternative pathway activation and the fate of such surfaceattached C3b is determined by the surface composition of the particle. Surface molecules influence whether a particle serves as activator or nonactivator of the pathway (Kazatchkine et al., 1979). Thus, a delicate balance determines the fate of the newly attached C3b molecules as to whether amplification occurs or inactivation by regulators and surface-bound inactivators is induced. It seems that the affinity of particle-bound C3b to its regulators, reconectin/FHL-1 and factor H, is a central checkpoint for this decision. In particular, surface components like polyanions and sialic acids determine the affinity of C3b molecules and C3bBb complexes for the negative regulators factor H and reconectin/FHL-1 and, thus, directly influence the decision towards activation or inactivation. Biological Consequences of Complement Activation Independent of its activation pathway, an amplified complement cascade leads to three actions: (1) Deposition C3 FI Cofactor activity Decay-acceleration activity FHL-1/Reconectin C3 Amplification FD Ba iC3b C3b C3b C3b C3b C3b C3b P Inactivation B Bb Bb Membrane Factor H Opsonization, Cell lysis C3b Figure 1 Formation and control of C3 at the cell surface. C3 is continuously activated by the alternative complement pathway and attaches to surfaces in the formof C3bin a random manner.Whenactivationoccurs, factorB (B)binds to C3b and is subsequentlycleaved by factor D (D) into fragmentBb and Ba. The resulting C3 convertase (C3bBb) is stabilized by binding of properdin (P) and, in an amplification loop, generates more C3b molecules which deposit in the vicinity of the convertase. A powerful amplification reaction results in surface deposition of C3b (opsonization) and formation of C5 convertases, followed by the induction of the lytic complement pathway and cell lysis. Complement regulators existing in the fluid phase and on the cell membrane determine the fate of the newly formed C3b molecule. The activity is shown here for the fluid-phase regulators factor H and reconectin/FHL-1. These regulators either direct the dissociation of the C3/C5 convertases, or they inhibit the formation of these convertases by controlling factor B binding, i.e. decay-accelerating activity. In addition, they act as cofactors for factor I and cause the inactivation of the C3b protein to the inactive form iC3b. Complement: Alternative Pathway 2 ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net