正在加载图片...
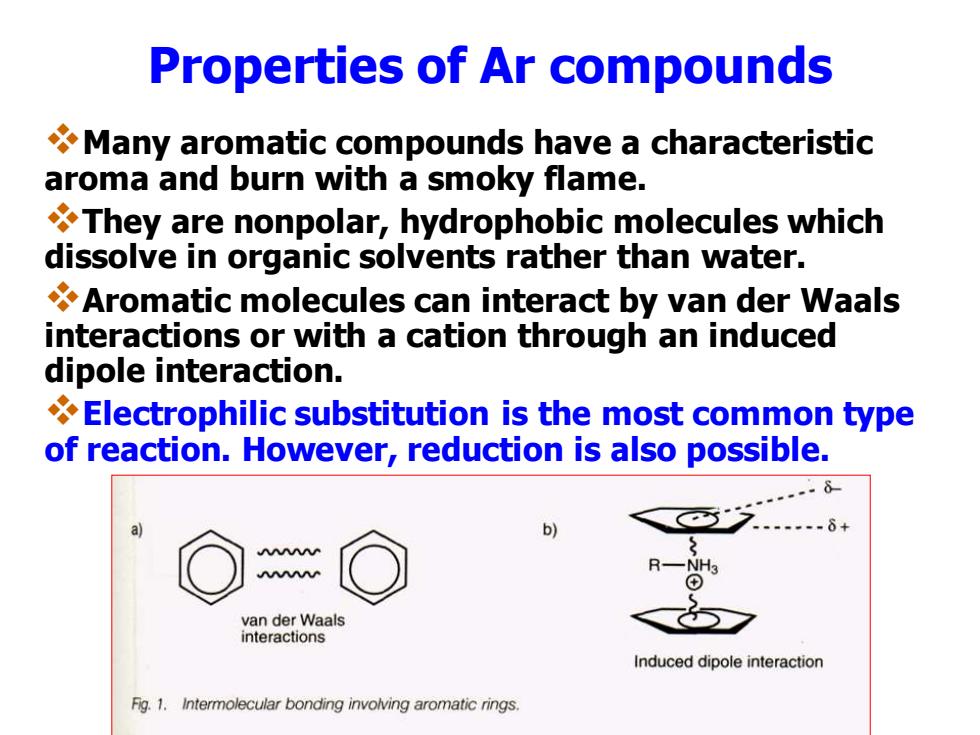
Properties of Ar compounds Many aromatic compounds have a characteristic aroma and burn with a smoky flame. They are nonpolar,hydrophobic molecules which dissolve in organic solvents rather than water. Aromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. Electrophilic substitution is the most common type of reaction.However,reduction is also possible. b) van der Waals interactions Induced dipole interaction Fig.1.Intermolecular bonding involving aromatic rings. Properties of Ar compounds ❖Many aromatic compounds have a characteristic aroma and burn with a smoky flame. ❖They are nonpolar, hydrophobic molecules which dissolve in organic solvents rather than water. ❖Aromatic molecules can interact by van der Waals interactions or with a cation through an induced dipole interaction. ❖Electrophilic substitution is the most common type of reaction. However, reduction is also possible