正在加载图片...
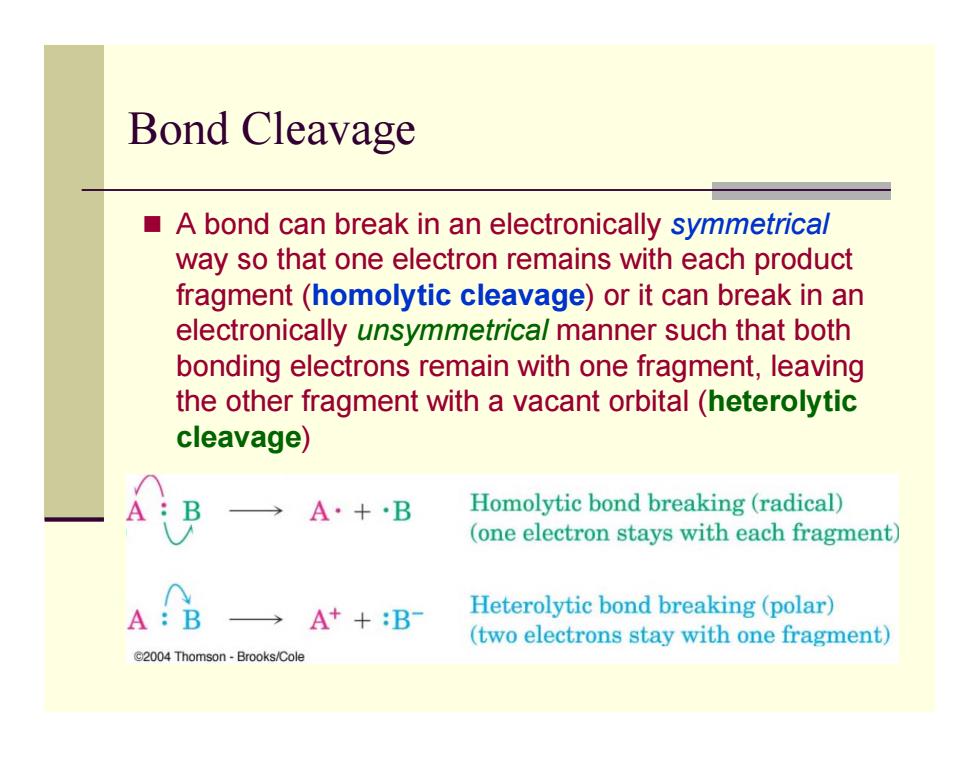
Bond Cleavage A bond can break in an electronically symmetrical way so that one electron remains with each product fragment (homolytic cleavage)or it can break in an electronically unsymmetrica/manner such that both bonding electrons remain with one fragment,leaving the other fragment with a vacant orbital (heterolytic cleavage) A·+B Homolytic bond breaking(radical) (one electron stays with each fragment) A:B →A++B Heterolytic bond breaking(polar) (two electrons stay with one fragment) C2004 Thomson-Brooks/Cole Bond Cleavage A bond can break in an electronically symmetrical way so that one electron remains with each product fragment (homolytic cleavage) or it can break in an electronically unsymmetrical manner such that both bonding electrons remain with one fragment, leaving the other fragment with a vacant orbital (heterolytic cleavage)