正在加载图片...
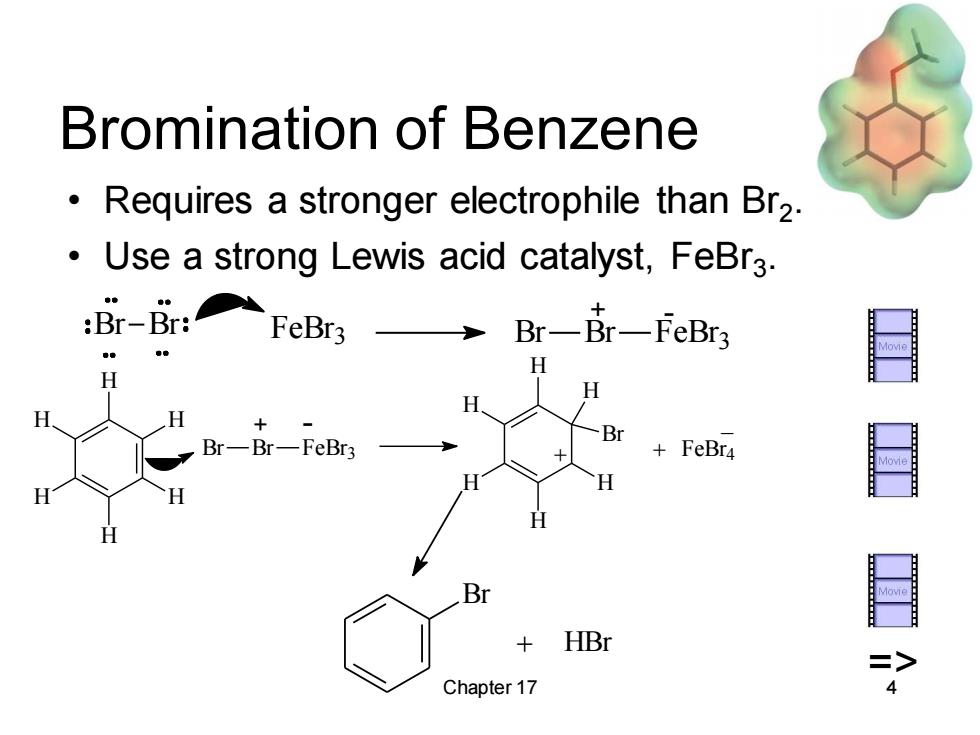
Bromination of Benzene Requires a stronger electrophile than Br2. Use a strong Lewis acid catalyst,FeBr3. :Br-Br:FeBr3 Br-Br一feBr3 H H H H H Br-Br-FeBr3 +FeBr4 H H H H H B + HBr Chapter 17 Chapter 17 4 Bromination of Benzene • Requires a stronger electrophile than Br2 . • Use a strong Lewis acid catalyst, FeBr3 . Br + HBr Br Br FeBr 3 Br Br FeBr 3 + - Br Br FeBr3 H H H H H H H H H H H H Br + + FeBr4 + - _ =>