正在加载图片...
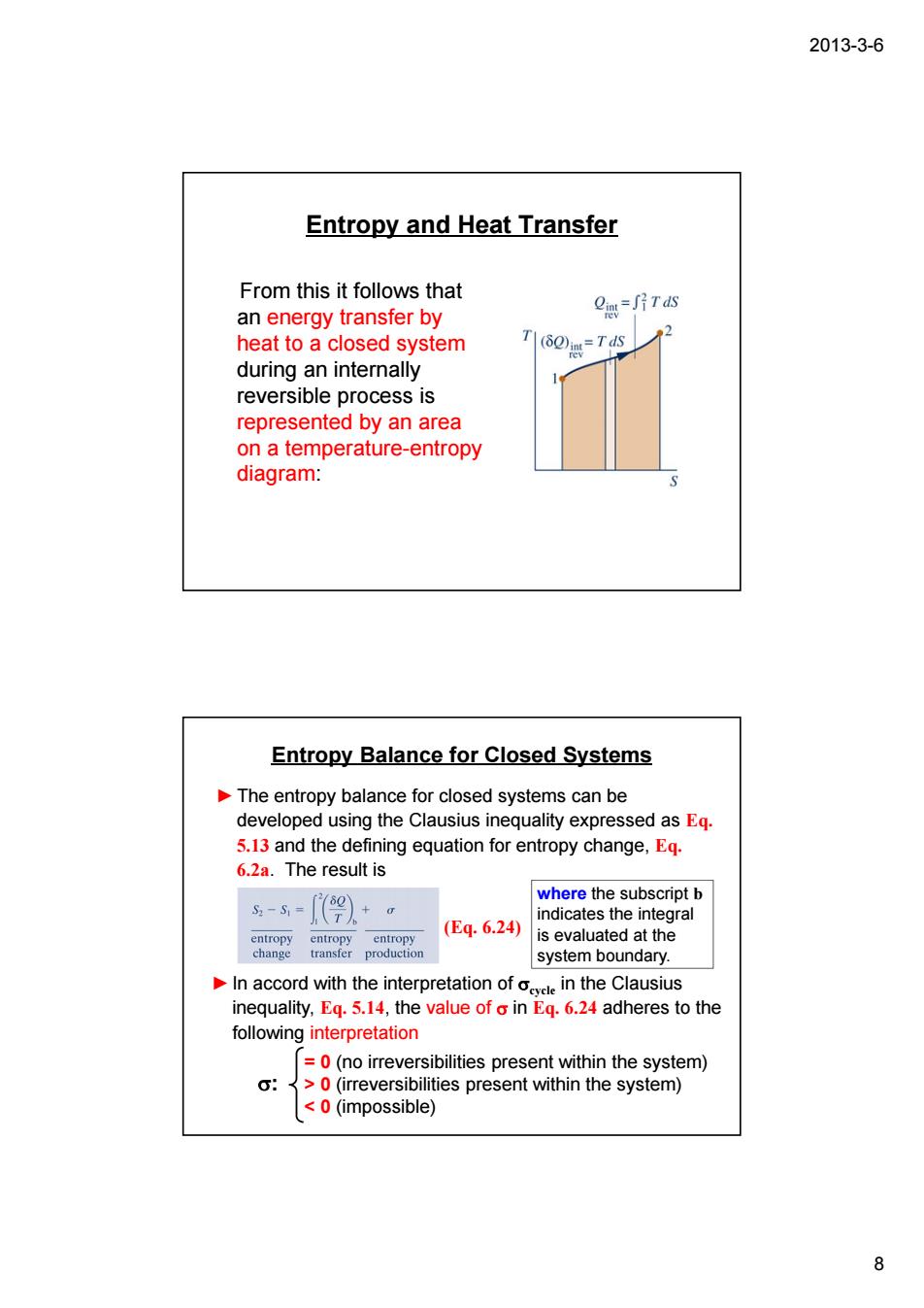
2013-3-6 Entropy and Heat Transfer From this it follows that an energy transfer by e=j片rds heat to a closed system T (6m=Td during an internally reversible process is represented by an area on a temperature-entropy diagram: Entropy Balance for Closed Systems The entropy balance for closed systems can be developed using the Clausius inequality expressed as Eq. 5.13 and the defining equation for entropy change,Eg. 6.2a The result is -=9》+。 (Eq.6.24 is evaluated at the system boundary. In accord with the interpretation ofin the Clausius inequality,Eq.5.14,the value of o in Eq.6.24 adheres to the following interpretation nirrbliiespresthn the system) ties present within the system) <0(impossible) 8 2013-3-6 8 Entropy and Heat Transfer From this it follows that an energy transfer by heat to a closed system during an internally reversible process is represented by an area on a temperature-entropy diagram: Entropy Balance for Closed Systems ►The entropy balance for closed systems can be developed using the Clausius inequality expressed as Eq. 5.13 and the defining equation for entropy change, Eq. 6.2a. The result is (Eq. 6.24) ►In accord with the interpretation of σcycle in the Clausius inequality, Eq. 5.14, the value of σ in Eq. 6.24 adheres to the following interpretation = 0 (no irreversibilities present within the system) > 0 (irreversibilities present within the system) < 0 (impossible) σ: where the subscript b indicates the integral is evaluated at the system boundary