正在加载图片...
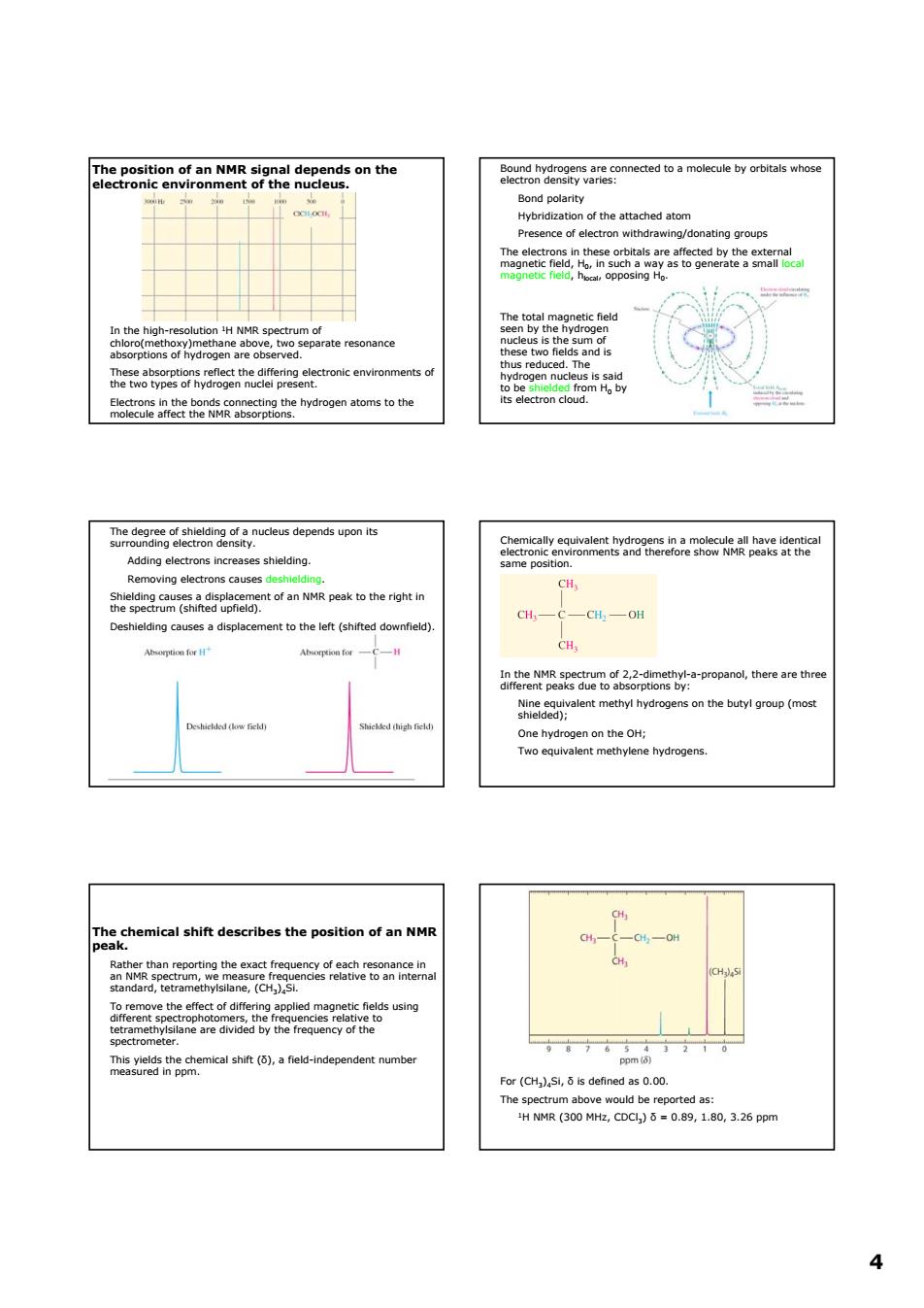
The position of an NMR signal depends on the the atached atom esence of electron withdrawing/donating groups ate resonance hetedyeearfvsbegnheaniectoncenmvtroament depends 2尚 cH, ntof an NMR ak to the riht in CHCCH,OH ent to the left (shifted downfield CH shle) on the butyl oroup (mos One hydrogen on theH; wo equ chemical shift describes the position of an NMP ane, using For(is defined as0. 4 4 The position of an NMR signal depends on the electronic environment of the nucleus. In the high-resolution 1H NMR spectrum of chloro(methoxy)methane above, two separate resonance absorptions of hydrogen are observed. These absorptions reflect the differing electronic environments of the two types of hydrogen nuclei present. Electrons in the bonds connecting the hydrogen atoms to the molecule affect the NMR absorptions. Bound hydrogens are connected to a molecule by orbitals whose electron density varies: Bond polarity Hybridization of the attached atom Presence of electron withdrawing/donating groups The electrons in these orbitals are affected by the external magnetic field, H0, in such a way as to generate a small local magnetic field, hlocal, opposing H0. The total magnetic field seen by the hydrogen nucleus is the sum of these two fields and is thus reduced. The hydrogen nucleus is said to be shielded from H0 by its electron cloud. The degree of shielding of a nucleus depends upon its surrounding electron density. Adding electrons increases shielding. Removing electrons causes deshielding. Shielding causes a displacement of an NMR peak to the right in the spectrum (shifted upfield). Deshielding causes a displacement to the left (shifted downfield). Chemically equivalent hydrogens in a molecule all have identical electronic environments and therefore show NMR peaks at the same position. In the NMR spectrum of 2,2-dimethyl-a-propanol, there are three different peaks due to absorptions by: Nine equivalent methyl hydrogens on the butyl group (most shielded); One hydrogen on the OH; Two equivalent methylene hydrogens. The chemical shift describes the position of an NMR peak. Rather than reporting the exact frequency of each resonance in an NMR spectrum, we measure frequencies relative to an internal standard, tetramethylsilane, (CH3)4Si. To remove the effect of differing applied magnetic fields using different spectrophotomers, the frequencies relative to tetramethylsilane are divided by the frequency of the spectrometer. This yields the chemical shift (δ), a field-independent number measured in ppm. For (CH3)4Si, δ is defined as 0.00. The spectrum above would be reported as: 1H NMR (300 MHz, CDCl3) δ = 0.89, 1.80, 3.26 ppm