正在加载图片...
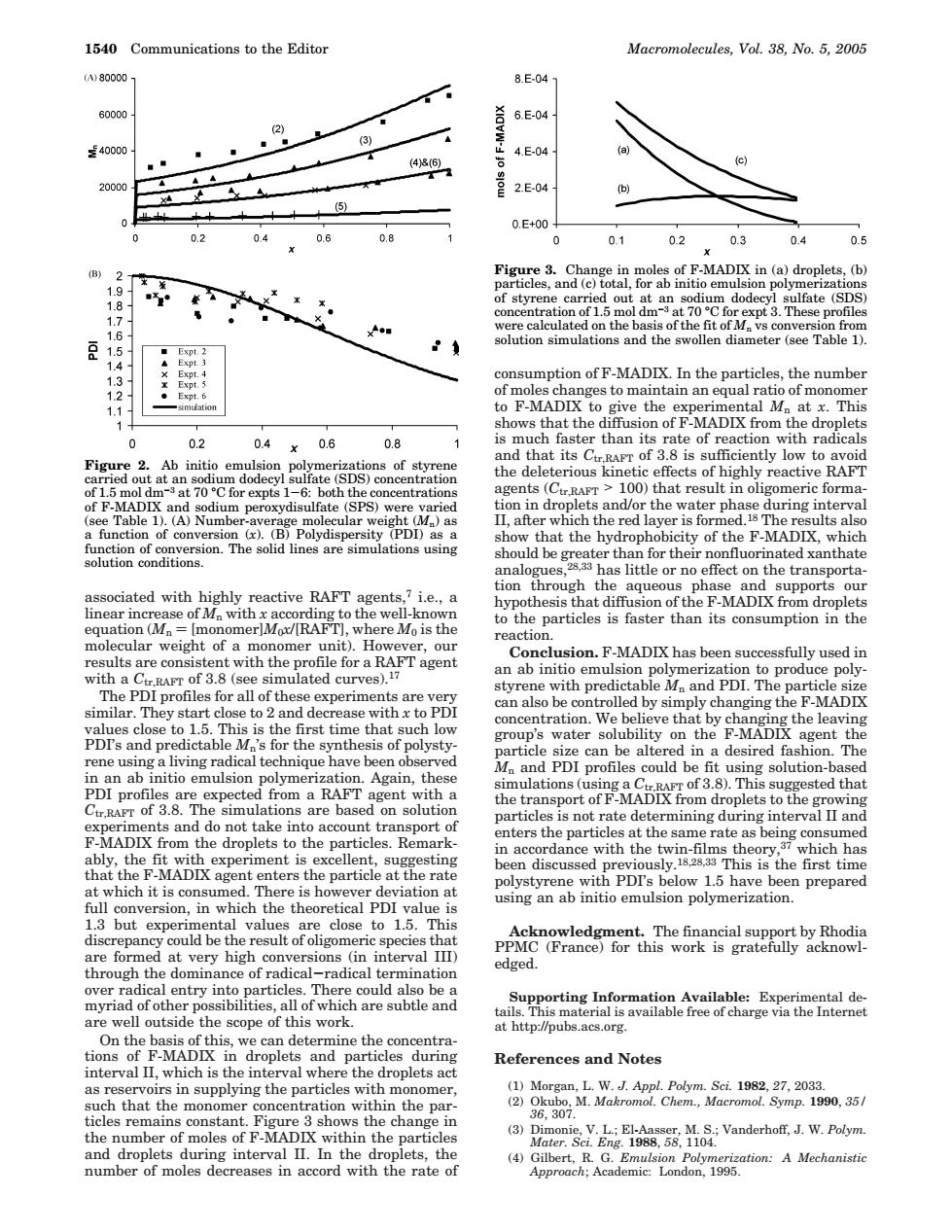
1540 Communications to the Edito Macromolecules,Vol.38.No.5.200 00 8E-04 6000 6E.0 3) 24000 4E0 2E04 0.E+0 02 04 0.8 0 01 0.2 0.3 0.4 05 B d a of 1.5 mol dmat 70C (SDS and the to m F-MADIX 02 04 0.6 0.8 1s m that it 2 of F-MADIX t70 A)Number-av ater ph erage ar olydi r that the hydr rophobicity of the F-MADIX,which hould beha tion support i.e.,a react onclusion.F-MADIX has been successfully used in ulated curves and dep ase with to can also be lled by simpl changing the F-MADLX water solubility on heF-ND Again.th M and PDI profiles could be f particles terval Ian no por sting in ace rda ciment i ce with the umed e iation a below 1.5 have n prepared 1.360 version, val through the domina of radical radical edged. ting Info mation Available:Exr cope of droplets and particles References and Notes aniannpnghepaidesoihm pa s the char 3) plets during interval II.In the th number of moles decreases in accord with the rate of associated with highly reactive RAFT agents,7 i.e., a linear increase of Mn with x according to the well-known equation (Mn ) [monomer]M0x/[RAFT], where M0 is the molecular weight of a monomer unit). However, our results are consistent with the profile for a RAFT agent with a Ctr,RAFT of 3.8 (see simulated curves).17 The PDI profiles for all of these experiments are very similar. They start close to 2 and decrease with x to PDI values close to 1.5. This is the first time that such low PDI’s and predictable Mn’s for the synthesis of polystyrene using a living radical technique have been observed in an ab initio emulsion polymerization. Again, these PDI profiles are expected from a RAFT agent with a Ctr,RAFT of 3.8. The simulations are based on solution experiments and do not take into account transport of F-MADIX from the droplets to the particles. Remarkably, the fit with experiment is excellent, suggesting that the F-MADIX agent enters the particle at the rate at which it is consumed. There is however deviation at full conversion, in which the theoretical PDI value is 1.3 but experimental values are close to 1.5. This discrepancy could be the result of oligomeric species that are formed at very high conversions (in interval III) through the dominance of radical-radical termination over radical entry into particles. There could also be a myriad of other possibilities, all of which are subtle and are well outside the scope of this work. On the basis of this, we can determine the concentrations of F-MADIX in droplets and particles during interval II, which is the interval where the droplets act as reservoirs in supplying the particles with monomer, such that the monomer concentration within the particles remains constant. Figure 3 shows the change in the number of moles of F-MADIX within the particles and droplets during interval II. In the droplets, the number of moles decreases in accord with the rate of consumption of F-MADIX. In the particles, the number of moles changes to maintain an equal ratio of monomer to F-MADIX to give the experimental Mn at x. This shows that the diffusion of F-MADIX from the droplets is much faster than its rate of reaction with radicals and that its Ctr,RAFT of 3.8 is sufficiently low to avoid the deleterious kinetic effects of highly reactive RAFT agents (Ctr,RAFT > 100) that result in oligomeric formation in droplets and/or the water phase during interval II, after which the red layer is formed.18 The results also show that the hydrophobicity of the F-MADIX, which should be greater than for their nonfluorinated xanthate analogues,28,33 has little or no effect on the transportation through the aqueous phase and supports our hypothesis that diffusion of the F-MADIX from droplets to the particles is faster than its consumption in the reaction. Conclusion. F-MADIX has been successfully used in an ab initio emulsion polymerization to produce polystyrene with predictable Mn and PDI. The particle size can also be controlled by simply changing the F-MADIX concentration. We believe that by changing the leaving group’s water solubility on the F-MADIX agent the particle size can be altered in a desired fashion. The Mn and PDI profiles could be fit using solution-based simulations (using a Ctr,RAFT of 3.8). This suggested that the transport of F-MADIX from droplets to the growing particles is not rate determining during interval II and enters the particles at the same rate as being consumed in accordance with the twin-films theory,37 which has been discussed previously.18,28,33 This is the first time polystyrene with PDI’s below 1.5 have been prepared using an ab initio emulsion polymerization. Acknowledgment. The financial support by Rhodia PPMC (France) for this work is gratefully acknowledged. Supporting Information Available: Experimental details. This material is available free of charge via the Internet at http://pubs.acs.org. References and Notes (1) Morgan, L. W. J. Appl. Polym. Sci. 1982, 27, 2033. (2) Okubo, M. Makromol. Chem., Macromol. Symp. 1990, 35/ 36, 307. (3) Dimonie, V. L.; El-Aasser, M. S.; Vanderhoff, J. W. Polym. Mater. Sci. Eng. 1988, 58, 1104. (4) Gilbert, R. G. Emulsion Polymerization: A Mechanistic Approach; Academic: London, 1995. Figure 2. Ab initio emulsion polymerizations of styrene carried out at an sodium dodecyl sulfate (SDS) concentration of 1.5 mol dm-3 at 70 °C for expts 1-6: both the concentrations of F-MADIX and sodium peroxydisulfate (SPS) were varied (see Table 1). (A) Number-average molecular weight (Mn) as a function of conversion (x). (B) Polydispersity (PDI) as a function of conversion. The solid lines are simulations using solution conditions. Figure 3. Change in moles of F-MADIX in (a) droplets, (b) particles, and (c) total, for ab initio emulsion polymerizations of styrene carried out at an sodium dodecyl sulfate (SDS) concentration of 1.5 mol dm-3 at 70 °C for expt 3. These profiles were calculated on the basis of the fit of Mn vs conversion from solution simulations and the swollen diameter (see Table 1). 1540 Communications to the Editor Macromolecules, Vol. 38, No. 5, 2005