正在加载图片...
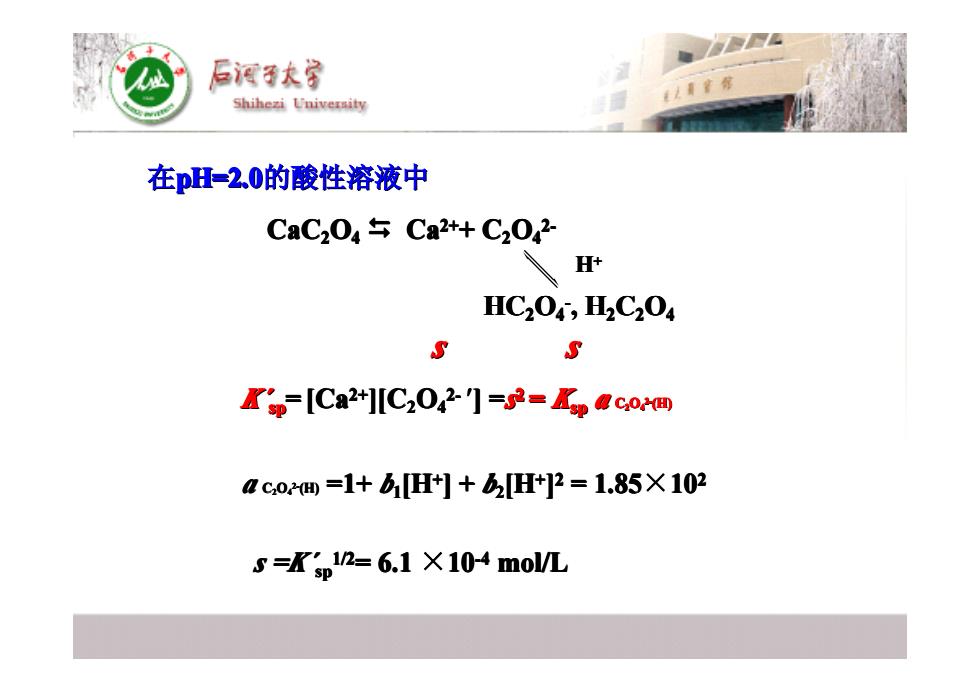
后酒子大 Shihezi University 在pH=2.0的酸性溶液中 CaC204午Ca2+C202 H+ HC204,H2C204 S S Kp-[Ca2+][C2042门=53=paco间 co=1+bH]+2H]2=1.85X102 5=Kp12-6.1X104mo/L 在pH=2.0的酸性溶液中 CaC22O44 � Ca2+2++ C22O44 2-2- s s s s s s s s s s s s s s s s HC22O44 -, H22C22O44 K´ spsp = [Ca2+2+ ][C22O44 2- 2-′] =s2 2= Kspsp a C22O44 2-2-(H) H++ a C22O44 2-2-(H) =1+ b11[H++ ] + b22[H++ ] 22 = 1.85×1022 s =K´ spsp 1/2 1/2= 6.1 ×10-4-4 mol/L