正在加载图片...
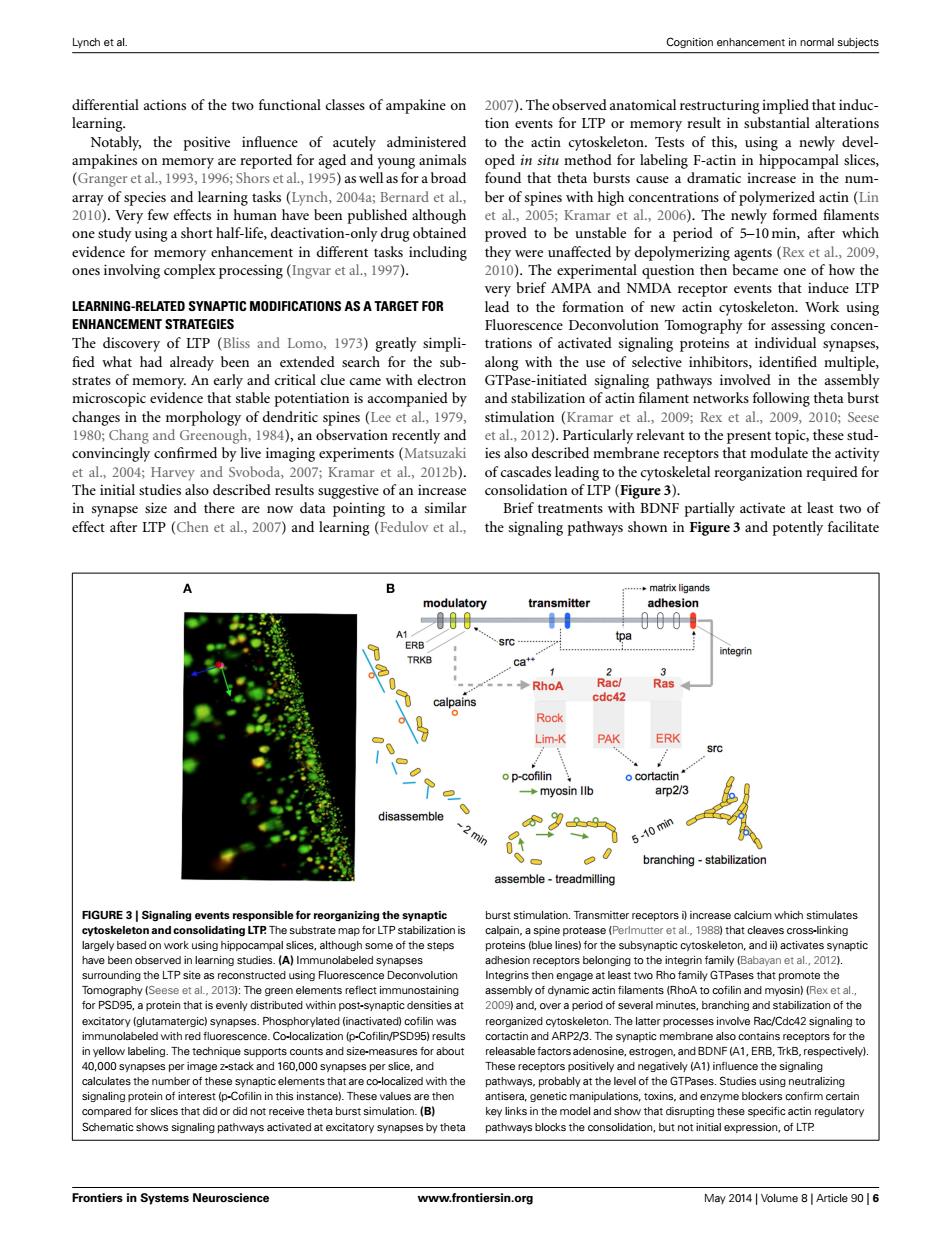
Lynch et al. Cognition enhancement in normal subjects differential actions of the two functional classes of ampakine on 2007).The observed anatomical restructuring implied that induc- learning. tion events for LTP or memory result in substantial alterations Notably,the positive influence of acutely administered to the actin cytoskeleton.Tests of this,using a newly devel- ampakines on memory are reported for aged and young animals oped in situ method for labeling F-actin in hippocampal slices, (Granger et al.,1993,1996;Shors et al,1995)as wellas for a broad found that theta bursts cause a dramatic increase in the num- array of species and learning tasks (Lynch,2004a;Bernard et al., ber of spines with high concentrations of polymerized actin (Lin 2010).Very few effects in human have been published although et al.,2005;Kramar et al.,2006).The newly formed filaments one study using a short half-life,deactivation-only drug obtained proved to be unstable for a period of 5-10min,after which evidence for memory enhancement in different tasks including they were unaffected by depolymerizing agents(Rex et al.,2009, ones involving complex processing (Ingvar et al.,1997). 2010).The experimental question then became one of how the very brief AMPA and NMDA receptor events that induce LTP LEARNING-RELATED SYNAPTIC MODIFICATIONS AS A TARGET FOR lead to the formation of new actin cytoskeleton.Work using ENHANCEMENT STRATEGIES Fluorescence Deconvolution Tomography for assessing concen- The discovery of LTP (Bliss and Lomo,1973)greatly simpli-trations of activated signaling proteins at individual synapses, fied what had already been an extended search for the sub-along with the use of selective inhibitors,identified multiple, strates of memory.An early and critical clue came with electron GTPase-initiated signaling pathways involved in the assembly microscopic evidence that stable potentiation is accompanied by and stabilization of actin filament networks following theta burst changes in the morphology of dendritic spines(Lee et al,1979, stimulation (Kramar et al.,2009;Rex et al.,2009,2010;Seese 1980;Chang and Greenough,1984),an observation recently and et al.,2012).Particularly relevant to the present topic,these stud- convincingly confirmed by live imaging experiments(Matsuzaki ies also described membrane receptors that modulate the activity et al.,2004;Harvey and Svoboda,2007;Kramar et al.,2012b).of cascades leading to the cytoskeletal reorganization required for The initial studies also described results suggestive of an increase consolidation of LTP (Figure 3). in synapse size and there are now data pointing to a similar Brief treatments with BDNF partially activate at least two of effect after LTP(Chen et al,2007)and learning(Fedulov et al., the signaling pathways shown in Figure 3 and potently facilitate ..matrix ligands modulatory transmitter adhesion 000 A1 tpa ERB integrin TRKB Ca+ -◆RhoA Racl Ras calpains cdc42 Rock Lim-K PAK ERK src o p-cofilin o cortactin ◆myosin llb arp2/3 disassemble 9 min 5-10 min branching-stabilization assemble-treadmilling FIGURE 3|Signaling events responsible for reorganizing the synaptic burst stimulation.Transmitter receptors i increase calcium which stimulates cytoskeleton and consolidating LTP.The substrate map for LTP stabilization is calpain,a spine protease (Perlmutter et al,1988)that cleaves cross-linking largely based on work using hippocampal slices,although some of the steps proteins (blue lines)for the subsynaptic cytoskeleton,and ii)activates synaptic have been observed in learning studies.(A)Immunolabeled synapses adhesion receptors belonging to the integrin family (Babayan et al.,2012). surrounding the LTP site as reconstructed using Fluorescence Deconvolution Integrins then engage at least two Rho family GTPases that promote the Tomography(Seese et al.,2013):The green elements reflect immunostaining assembly of dynamic actin filaments (RhoA to cofilin and myosin)(Rex et al., for PSD95,a protein that is evenly distributed within post-synaptic densities at 2009)and,over a period of several minutes,branching and stabilization of the excitatory (glutamatergic)synapses.Phosphorylated (inactivated)cofilin was reorganized cytoskeleton.The latter processes involve Rac/Cdc42 signaling to immunolabeled with red fluorescence.Co-localization (p-Cofilin/PSD95)results cortactin and ARP2/3.The synaptic membrane also contains receptors for the in yellow labeling.The technique supports counts and size-measures for about releasable factors adenosine,estrogen,and BDNF(A1,ERB,TrkB,respectively) 40,000 synapses per image z-stack and 160,000 synapses per slice,and These receptors positively and negatively(A1)influence the signaling calculates the number of these synaptic elements that are co-localized with the pathways,probably at the level of the GTPases.Studies using neutralizing signaling protein of interest (p-Cofilin in this instance).These values are then antisera,genetic manipulations,toxins,and enzyme blockers confirm certain compared for slices that did or did not receive theta burst simulation.(B) key links in the model and show that disrupting these specific actin regulatory Schematic shows signaling pathways activated at excitatory synapses by theta pathways blocks the consolidation,but not initial expression,of LTP Frontiers in Systems Neuroscience www.frontiersin.org May 2014 Volume 8 Article 90 6Lynch et al. Cognition enhancement in normal subjects differential actions of the two functional classes of ampakine on learning. Notably, the positive influence of acutely administered ampakines on memory are reported for aged and young animals (Granger et al., 1993, 1996; Shors et al., 1995) as well as for a broad array of species and learning tasks (Lynch, 2004a; Bernard et al., 2010). Very few effects in human have been published although one study using a short half-life, deactivation-only drug obtained evidence for memory enhancement in different tasks including ones involving complex processing (Ingvar et al., 1997). LEARNING-RELATED SYNAPTIC MODIFICATIONS AS A TARGET FOR ENHANCEMENT STRATEGIES The discovery of LTP (Bliss and Lomo, 1973) greatly simpli- fied what had already been an extended search for the substrates of memory. An early and critical clue came with electron microscopic evidence that stable potentiation is accompanied by changes in the morphology of dendritic spines (Lee et al., 1979, 1980; Chang and Greenough, 1984), an observation recently and convincingly confirmed by live imaging experiments (Matsuzaki et al., 2004; Harvey and Svoboda, 2007; Kramar et al., 2012b). The initial studies also described results suggestive of an increase in synapse size and there are now data pointing to a similar effect after LTP (Chen et al., 2007) and learning (Fedulov et al., 2007). The observed anatomical restructuring implied that induction events for LTP or memory result in substantial alterations to the actin cytoskeleton. Tests of this, using a newly developed in situ method for labeling F-actin in hippocampal slices, found that theta bursts cause a dramatic increase in the number of spines with high concentrations of polymerized actin (Lin et al., 2005; Kramar et al., 2006). The newly formed filaments proved to be unstable for a period of 5–10 min, after which they were unaffected by depolymerizing agents (Rex et al., 2009, 2010). The experimental question then became one of how the very brief AMPA and NMDA receptor events that induce LTP lead to the formation of new actin cytoskeleton. Work using Fluorescence Deconvolution Tomography for assessing concentrations of activated signaling proteins at individual synapses, along with the use of selective inhibitors, identified multiple, GTPase-initiated signaling pathways involved in the assembly and stabilization of actin filament networks following theta burst stimulation (Kramar et al., 2009; Rex et al., 2009, 2010; Seese et al., 2012). Particularly relevant to the present topic, these studies also described membrane receptors that modulate the activity of cascades leading to the cytoskeletal reorganization required for consolidation of LTP (Figure 3). Brief treatments with BDNF partially activate at least two of the signaling pathways shown in Figure 3 and potently facilitate FIGURE 3 | Signaling events responsible for reorganizing the synaptic cytoskeleton and consolidating LTP. The substrate map for LTP stabilization is largely based on work using hippocampal slices, although some of the steps have been observed in learning studies. (A) Immunolabeled synapses surrounding the LTP site as reconstructed using Fluorescence Deconvolution Tomography (Seese et al., 2013): The green elements reflect immunostaining for PSD95, a protein that is evenly distributed within post-synaptic densities at excitatory (glutamatergic) synapses. Phosphorylated (inactivated) cofilin was immunolabeled with red fluorescence. Co-localization (p-Cofilin/PSD95) results in yellow labeling. The technique supports counts and size-measures for about 40,000 synapses per image z-stack and 160,000 synapses per slice, and calculates the number of these synaptic elements that are co-localized with the signaling protein of interest (p-Cofilin in this instance). These values are then compared for slices that did or did not receive theta burst simulation. (B) Schematic shows signaling pathways activated at excitatory synapses by theta burst stimulation. Transmitter receptors i) increase calcium which stimulates calpain, a spine protease (Perlmutter et al., 1988) that cleaves cross-linking proteins (blue lines) for the subsynaptic cytoskeleton, and ii) activates synaptic adhesion receptors belonging to the integrin family (Babayan et al., 2012). Integrins then engage at least two Rho family GTPases that promote the assembly of dynamic actin filaments (RhoA to cofilin and myosin) (Rex et al., 2009) and, over a period of several minutes, branching and stabilization of the reorganized cytoskeleton. The latter processes involve Rac/Cdc42 signaling to cortactin and ARP2/3. The synaptic membrane also contains receptors for the releasable factors adenosine, estrogen, and BDNF (A1, ERB, TrkB, respectively). These receptors positively and negatively (A1) influence the signaling pathways, probably at the level of the GTPases. Studies using neutralizing antisera, genetic manipulations, toxins, and enzyme blockers confirm certain key links in the model and show that disrupting these specific actin regulatory pathways blocks the consolidation, but not initial expression, of LTP. Frontiers in Systems Neuroscience www.frontiersin.org May 2014 | Volume 8 | Article 90 | 6