正在加载图片...
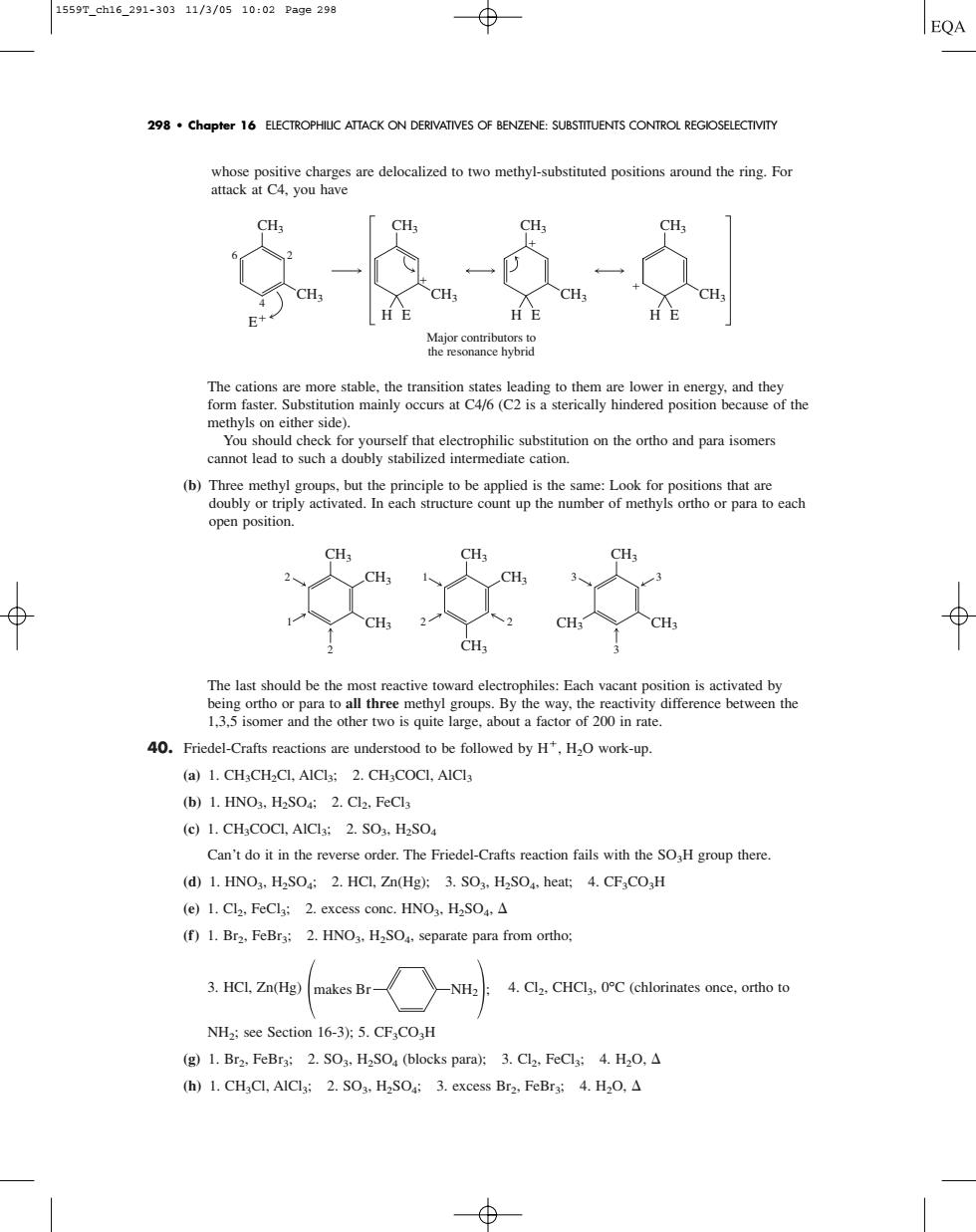
1559T_ch16_291-30311/3/0510:02Page298 ⊕ EQA 298.Chapter 16 ELECTROPHIUC ATTACK ON DERIVATIVES OF BENZENE:SUBSTITUENTS CONTROL REGIOSELECTIVITY ive cha are delocalized to two methyl-substituted positio round the ring.Fo attack at C4.you have Major o You should check for yourself that electrophilic substitution on the ortho and para isomers cannot lead to such a doubly stabilized intermediate cation. open position 女 1.3.5 isomer and the other two is quite large,about a factor of 200 in rate. 0.Friedel-Crafts reactions are understood to be followed byHHwork-up. (a)1.CH CH-CL.AICl:2.CHCOCI.AICla (b)1.HNO3.H2SO:2.Cl2.FeCls (e)1.CHaCOCI.AICla:2.SO3.H2SO Can't do it in the reverse order.The Friedel-Crafts reaction fails with the SOH group there (d)1.HNO.HSO:2.HCL.Zn(Hg):3.SO:.HSO.heat:4.CF COH (e)1.Cl2.FeCla:2.excess conc.HNO3.HaSO.A (f)1.Br2.FeBrs:2.HNO3.HSO.separate para from ortho: -NH2:4.Cl2.CHCla.C(chlorinates once.ortho to NHa;see Section 16-3):5.CF.CO,H (g)1.Br2.FeBra:2.SO3.H2SO(blocks para):3.Cl2.FeClg:4.H2O,A (h)1.CH,Cl,AIClg:2.SO3.H2SO:3.excess Bra.FeBrs:4.H2O.A whose positive charges are delocalized to two methyl-substituted positions around the ring. For attack at C4, you have The cations are more stable, the transition states leading to them are lower in energy, and they form faster. Substitution mainly occurs at C4/6 (C2 is a sterically hindered position because of the methyls on either side). You should check for yourself that electrophilic substitution on the ortho and para isomers cannot lead to such a doubly stabilized intermediate cation. (b) Three methyl groups, but the principle to be applied is the same: Look for positions that are doubly or triply activated. In each structure count up the number of methyls ortho or para to each open position. The last should be the most reactive toward electrophiles: Each vacant position is activated by being ortho or para to all three methyl groups. By the way, the reactivity difference between the 1,3,5 isomer and the other two is quite large, about a factor of 200 in rate. 40. Friedel-Crafts reactions are understood to be followed by H, H2O work-up. (a) 1. CH3CH2Cl, AlCl3; 2. CH3COCl, AlCl3 (b) 1. HNO3, H2SO4; 2. Cl2, FeCl3 (c) 1. CH3COCl, AlCl3; 2. SO3, H2SO4 Can’t do it in the reverse order. The Friedel-Crafts reaction fails with the SO3H group there. (d) 1. HNO3, H2SO4; 2. HCl, Zn(Hg); 3. SO3, H2SO4, heat; 4. CF3CO3H (e) 1. Cl2, FeCl3; 2. excess conc. HNO3, H2SO4, (f) 1. Br2, FeBr3; 2. HNO3, H2SO4, separate para from ortho; 3. HCl, Zn(Hg) 4. Cl2, CHCl3, 0°C (chlorinates once, ortho to NH2; see Section 16-3); 5. CF3CO3H (g) 1. Br2, FeBr3; 2. SO3, H2SO4 (blocks para); 3. Cl2, FeCl3; 4. H2O, (h) 1. CH3Cl, AlCl3; 2. SO3, H2SO4; 3. excess Br2, FeBr3; 4. H2O, makes Br NH2 ; CH3 CH3 1 2 2 CH3 CH3 1 2 CH3 2 CH3 3 3 CH3 3 CH3 CH3 E H E Major contributors to the resonance hybrid CH3 CH3 6 2 4 CH3 CH3 CH3 CH3 H E CH3 H E CH3 298 • Chapter 16 ELECTROPHILIC ATTACK ON DERIVATIVES OF BENZENE: SUBSTITUENTS CONTROL REGIOSELECTIVITY 1559T_ch16_291-303 11/3/05 10:02 Page 298�����