正在加载图片...
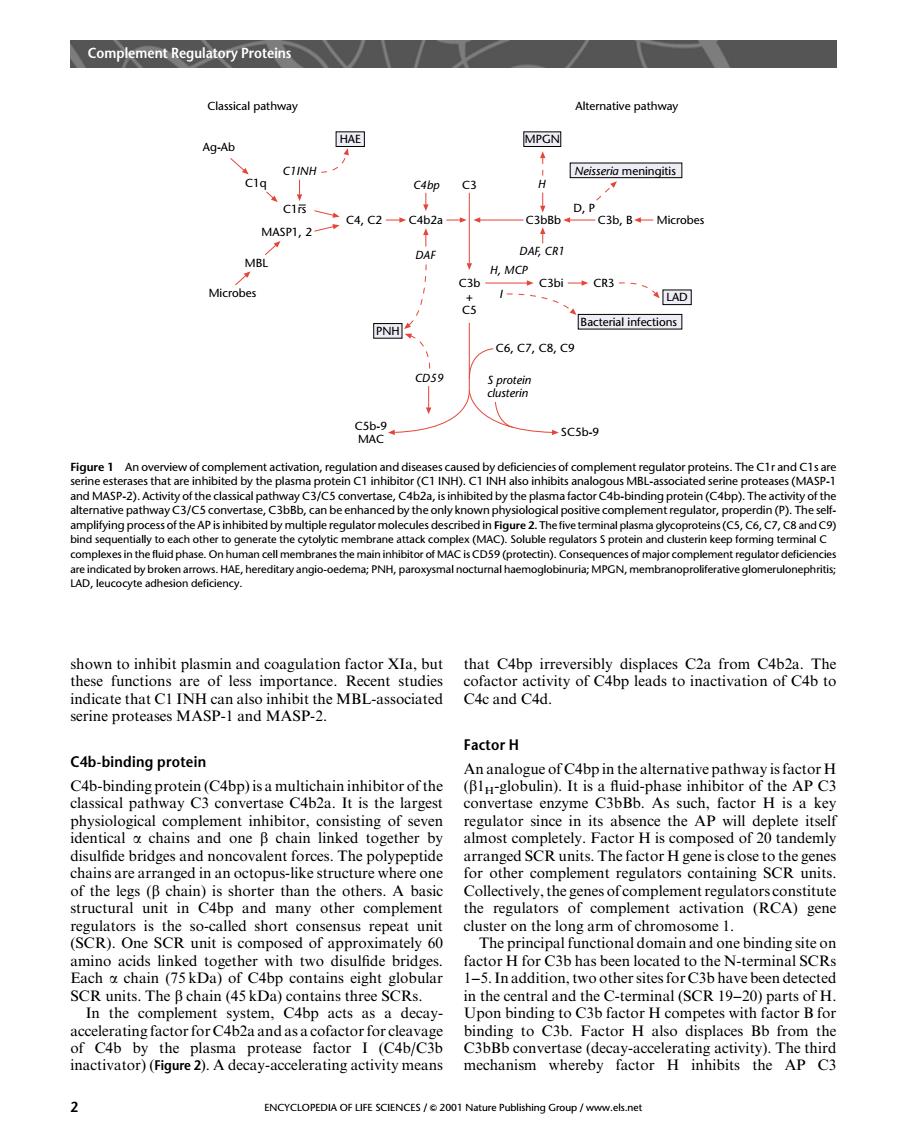
Complme ReguPrt Classical pathway Alternative pathwa Ag-Ab HAE MPGN CIINH C4bp C3 Neissermn▣ Msp1L.2c4,ac4b2a C3b8b-D.P C3b.BMicrobes DAF,CR1 C3b H,MCP CR3 1- “A可 PNH Bacterial infections C6,C7,C8.C9 CD59 5C5b-9 An CI in H INH ses (M to genera olub ors S prot bintgarrgHEheredtayango-oedemgPWNHpargynmalncC MPCN membranoproiferative glomerulonephritis Sofactor actvity o +bp serine proteases MASP-I and MASP-2. Factor H C4b-binding protein C4b-binding protein(C4bp)isa multichain inhibitor of the classical pathway C3 convertase C4b2a.It is the largest tootogical convertase enzyme C3bBb.As such,factor H is a key eplete itse anc chains are arranged in an octopus like structure where on for other complement regulators containine SCR units of the legs (B chain)is shorter than the others.A basic Collectively,the genes ofcomplement regulatorsconstitute structural unit in C4bp the regulators of complement activation (RCA)gene short ensus repea ster on the hromosome amino acids linked too ther with two disulfide bri achchan (Da)of Cbpht globula factor H for C3b has been located to the N-terminal SCRs 1-5.In addition,twoother sites for C3b have been detected SCR units.The B chain(45 kDa)contains three SC central and the C-terminal (SCR 19- In the comple C4bp acts as n b H rotease factor I (C4b/C3b inactivator)(Figure2).A deeay-accelerating activity means mechanism whereby factor H inhibits the AP C3 2 ENCYCLOPEDIA OF LIFE SCIENCES/2001Nashown to inhibit plasmin and coagulation factor XIa, but these functions are of less importance. Recent studies indicate that C1 INH can also inhibit the MBL-associated serine proteases MASP-1 and MASP-2. C4b-binding protein C4b-binding protein (C4bp) is a multichain inhibitor of the classical pathway C3 convertase C4b2a. It is the largest physiological complement inhibitor, consisting of seven identical a chains and one b chain linked together by disulfide bridges and noncovalent forces. The polypeptide chains are arranged in an octopus-like structure where one of the legs (b chain) is shorter than the others. A basic structural unit in C4bp and many other complement regulators is the so-called short consensus repeat unit (SCR). One SCR unit is composed of approximately 60 amino acids linked together with two disulfide bridges. Each a chain (75 kDa) of C4bp contains eight globular SCR units. The b chain (45 kDa) contains three SCRs. In the complement system, C4bp acts as a decayaccelerating factor for C4b2a and as a cofactor for cleavage of C4b by the plasma protease factor I (C4b/C3b inactivator) (Figure 2). A decay-accelerating activity means that C4bp irreversibly displaces C2a from C4b2a. The cofactor activity of C4bp leads to inactivation of C4b to C4c and C4d. Factor H An analogue of C4bp in the alternative pathway is factor H (b1H-globulin). It is a fluid-phase inhibitor of the AP C3 convertase enzyme C3bBb. As such, factor H is a key regulator since in its absence the AP will deplete itself almost completely. Factor H is composed of 20 tandemly arranged SCR units. The factor H gene is close to the genes for other complement regulators containing SCR units. Collectively, the genes of complement regulators constitute the regulators of complement activation (RCA) gene cluster on the long arm of chromosome 1. The principal functional domain and one binding site on factor H for C3b has been located to the N-terminal SCRs 1–5. In addition, two other sites for C3b have been detected in the central and the C-terminal (SCR 19–20) parts of H. Upon binding to C3b factor H competes with factor B for binding to C3b. Factor H also displaces Bb from the C3bBb convertase (decay-accelerating activity). The third mechanism whereby factor H inhibits the AP C3 Classical pathway Ag-Ab C1q C1rs C1INH MASP1, 2 MBL Microbes HAE C4, C2 C4b2a C4bp DAF PNH C3 CD59 C5b-9 MAC C3b + C5 C3bi CR3 I LAD Bacterial infections C6, C7, C8, C9 S protein clusterin SC5b-9 MPGN H C3bBb DAF, CR1 H, MCP C3b, B D, P Neisseria meningitis Microbes Alternative pathway Figure 1 An overview of complement activation, regulation and diseases caused by deficiencies of complement regulator proteins. The C1r and C1s are serine esterases that are inhibited by the plasma protein C1 inhibitor (C1 INH). C1 INH also inhibits analogous MBL-associated serine proteases (MASP-1 and MASP-2). Activity of the classical pathway C3/C5 convertase, C4b2a, is inhibited by the plasma factor C4b-binding protein (C4bp). The activity of the alternative pathway C3/C5 convertase, C3bBb, can be enhanced by the only known physiological positive complement regulator, properdin (P). The selfamplifying process of the AP is inhibited by multiple regulator molecules described in Figure 2. The five terminal plasma glycoproteins (C5, C6, C7, C8 and C9) bind sequentially to each other to generate the cytolytic membrane attack complex (MAC). Soluble regulators S protein and clusterin keep forming terminal C complexes in the fluid phase. On human cell membranes the main inhibitor of MAC is CD59 (protectin). Consequences of major complement regulator deficiencies are indicated by broken arrows. HAE, hereditary angio-oedema; PNH, paroxysmal nocturnal haemoglobinuria; MPGN, membranoproliferative glomerulonephritis; LAD, leucocyte adhesion deficiency. Complement Regulatory Proteins 2 ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Nature Publishing Group / www.els.net