正在加载图片...
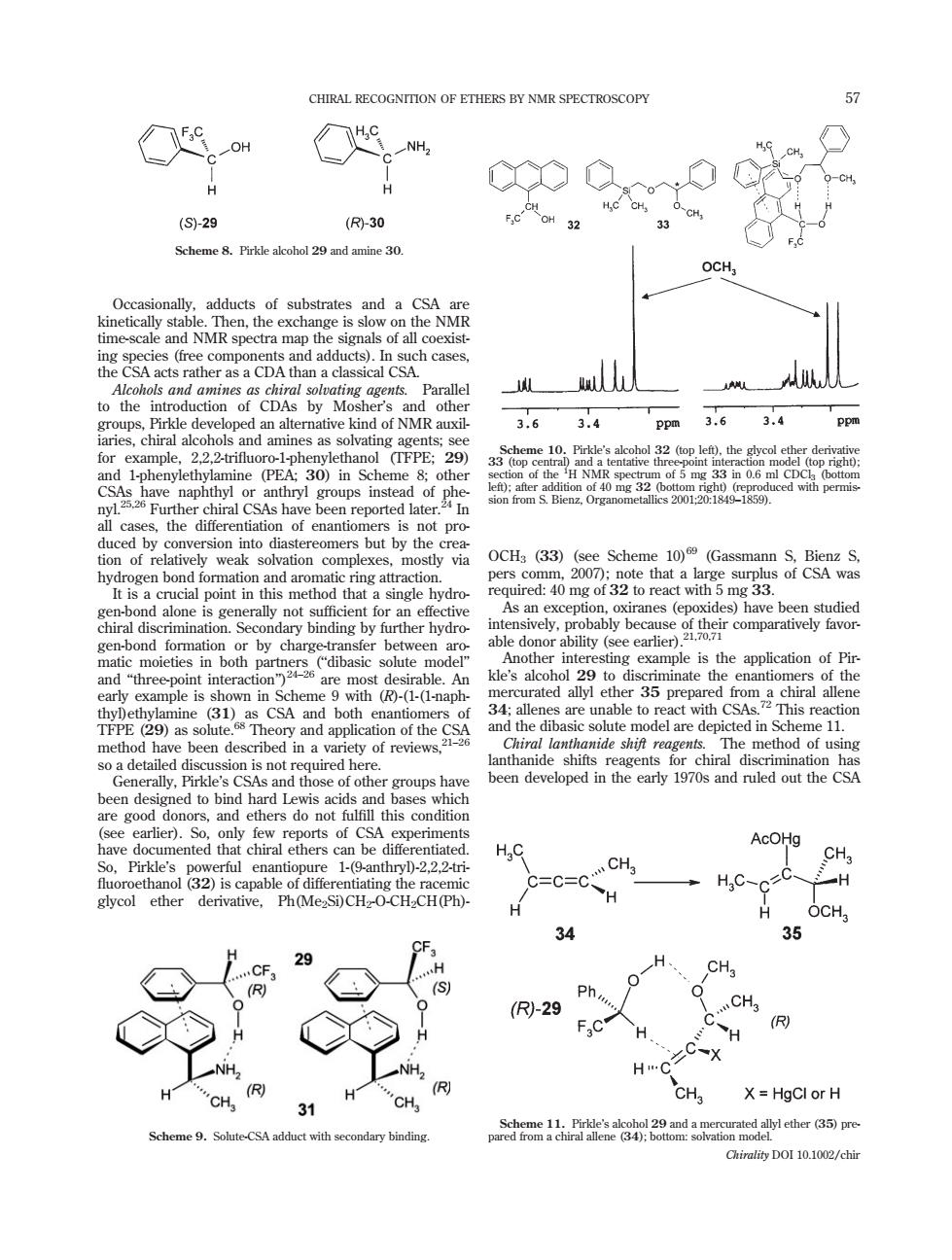
CHIRAL RECOGNITION OF ETHERS BY NMR SPECTROSCOPY 一OH ◇e NH2 CH 0- (S)-29 (R-30 OCH, me-sca acts rather as aCDA than a classical CSA to the introduction of CDAs by Mosher's MR se 3.6 3.4 Ppm 3.634 2meoeEhs0ehaol FPE: e 5 06 naphthyl or anthryl gen bond formatior ring attr not suficient for an efe iral allene eme 11 een des ribed in a y of review uns hav been developed in the early 1970s and ruled out the CSA en designed to bi do not fu H,C AcO CH Pirkle's rful enanti .222t CH与 s capab C=C ycol ether Ph(Me SOCH O-CH CH(Ph DCH 34 H、 0 (R)-29 R CH X=HgCl or H aa9am1r阿m Chirality DOI 10.1002/chirOccasionally, adducts of substrates and a CSA are kinetically stable. Then, the exchange is slow on the NMR time-scale and NMR spectra map the signals of all coexisting species (free components and adducts). In such cases, the CSA acts rather as a CDA than a classical CSA. Alcohols and amines as chiral solvating agents. Parallel to the introduction of CDAs by Mosher’s and other groups, Pirkle developed an alternative kind of NMR auxiliaries, chiral alcohols and amines as solvating agents; see for example, 2,2,2-trifluoro-1-phenylethanol (TFPE; 29) and 1-phenylethylamine (PEA; 30) in Scheme 8; other CSAs have naphthyl or anthryl groups instead of phenyl.25,26 Further chiral CSAs have been reported later.24 In all cases, the differentiation of enantiomers is not produced by conversion into diastereomers but by the creation of relatively weak solvation complexes, mostly via hydrogen bond formation and aromatic ring attraction. It is a crucial point in this method that a single hydrogen-bond alone is generally not sufficient for an effective chiral discrimination. Secondary binding by further hydrogen-bond formation or by charge-transfer between aromatic moieties in both partners (‘‘dibasic solute model’’ and ‘‘three-point interaction’’)24–26 are most desirable. An early example is shown in Scheme 9 with (R)-(1-(1-naphthyl)ethylamine (31) as CSA and both enantiomers of TFPE (29) as solute.68 Theory and application of the CSA method have been described in a variety of reviews,21–26 so a detailed discussion is not required here. Generally, Pirkle’s CSAs and those of other groups have been designed to bind hard Lewis acids and bases which are good donors, and ethers do not fulfill this condition (see earlier). So, only few reports of CSA experiments have documented that chiral ethers can be differentiated. So, Pirkle’s powerful enantiopure 1-(9-anthryl)-2,2,2-tri- fluoroethanol (32) is capable of differentiating the racemic glycol ether derivative, Ph(Me2Si)CH2-O-CH2CH(Ph)- OCH3 (33) (see Scheme 10)69 (Gassmann S, Bienz S, pers comm, 2007); note that a large surplus of CSA was required: 40 mg of 32 to react with 5 mg 33. As an exception, oxiranes (epoxides) have been studied intensively, probably because of their comparatively favorable donor ability (see earlier).21,70,71 Another interesting example is the application of Pirkle’s alcohol 29 to discriminate the enantiomers of the mercurated allyl ether 35 prepared from a chiral allene 34; allenes are unable to react with CSAs.72 This reaction and the dibasic solute model are depicted in Scheme 11. Chiral lanthanide shift reagents. The method of using lanthanide shifts reagents for chiral discrimination has been developed in the early 1970s and ruled out the CSA Scheme 8. Pirkle alcohol 29 and amine 30. Scheme 9. Solute-CSA adduct with secondary binding. Scheme 10. Pirkle’s alcohol 32 (top left), the glycol ether derivative 33 (top central) and a tentative three-point interaction model (top right); section of the 1 H NMR spectrum of 5 mg 33 in 0.6 ml CDCl3 (bottom left); after addition of 40 mg 32 (bottom right) (reproduced with permission from S. Bienz, Organometallics 2001;20:1849–1859). Scheme 11. Pirkle’s alcohol 29 and a mercurated allyl ether (35) prepared from a chiral allene (34); bottom: solvation model. CHIRAL RECOGNITION OF ETHERS BY NMR SPECTROSCOPY 57 Chirality DOI 10.1002/chir