正在加载图片...
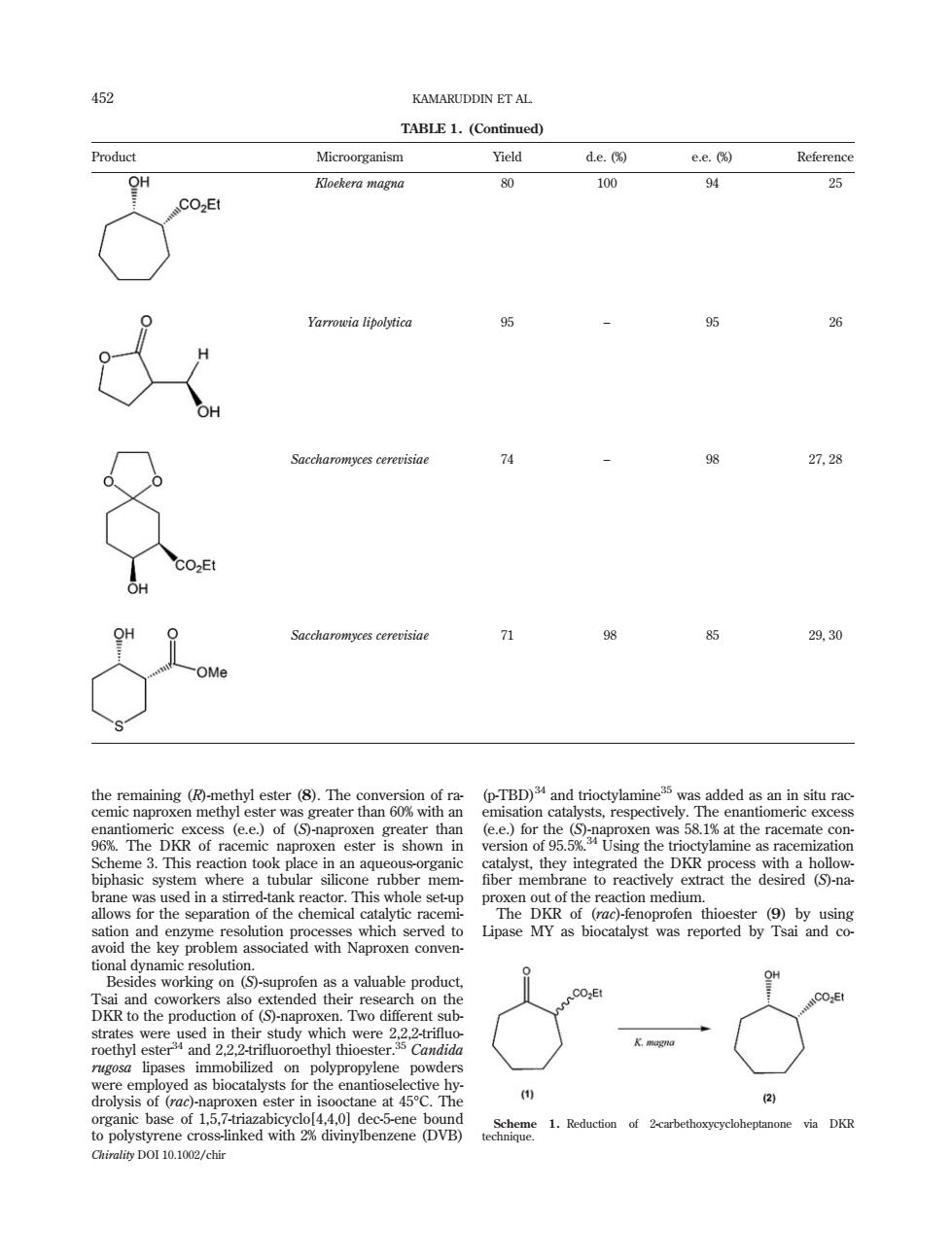
452 KAMARUDDIN ET AL TABLE 1.(Continued) Microorganism Yield de. ee Reference oe味era magna 100 94 25 Yarrowia lipolytica 26 Saccharomyces cerevisiae 74 27,28 71 29,30 of ra (p-TBD)and trioctylamine35 was added as an in situ rac hiaewacnatmedamtaacaTtitceoetp tional dynam esolutio c0 DKR to the production of()n Two diff 2 of 15.7 xen e ster i ne at 45 to polystyrene cr 1.Reduction of 2-carbethoxycycloheptanone via DKR Chirality DOI 10.1002/chir the remaining (R)-methyl ester (8). The conversion of racemic naproxen methyl ester was greater than 60% with an enantiomeric excess (e.e.) of (S)-naproxen greater than 96%. The DKR of racemic naproxen ester is shown in Scheme 3. This reaction took place in an aqueous-organic biphasic system where a tubular silicone rubber membrane was used in a stirred-tank reactor. This whole set-up allows for the separation of the chemical catalytic racemisation and enzyme resolution processes which served to avoid the key problem associated with Naproxen conventional dynamic resolution. Besides working on (S)-suprofen as a valuable product, Tsai and coworkers also extended their research on the DKR to the production of (S)-naproxen. Two different substrates were used in their study which were 2,2,2-trifluoroethyl ester34 and 2,2,2-trifluoroethyl thioester.35 Candida rugosa lipases immobilized on polypropylene powders were employed as biocatalysts for the enantioselective hydrolysis of (rac)-naproxen ester in isooctane at 458C. The organic base of 1,5,7-triazabicyclo[4,4,0] dec-5-ene bound to polystyrene cross-linked with 2% divinylbenzene (DVB) (p-TBD)34 and trioctylamine35 was added as an in situ racemisation catalysts, respectively. The enantiomeric excess (e.e.) for the (S)-naproxen was 58.1% at the racemate conversion of 95.5%.34 Using the trioctylamine as racemization catalyst, they integrated the DKR process with a hollow- fiber membrane to reactively extract the desired (S)-naproxen out of the reaction medium. The DKR of (rac)-fenoprofen thioester (9) by using Lipase MY as biocatalyst was reported by Tsai and coTABLE 1. (Continued) Product Microorganism Yield d.e. (%) e.e. (%) Reference Kloekera magna 80 100 94 25 Yarrowia lipolytica 95 – 95 26 Saccharomyces cerevisiae 74 – 98 27, 28 Saccharomyces cerevisiae 71 98 85 29, 30 Scheme 1. Reduction of 2-carbethoxycycloheptanone via DKR technique. 452 KAMARUDDIN ET AL. Chirality DOI 10.1002/chir