正在加载图片...
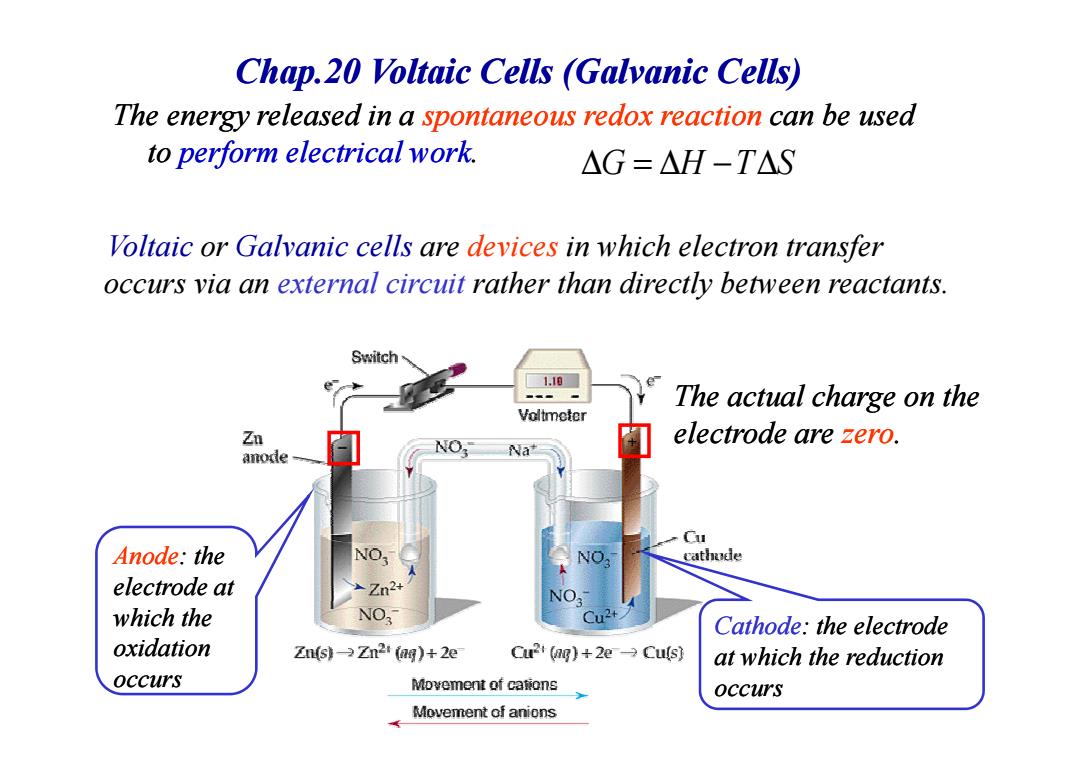
Chap.20 Voltaic Cells (Galvanic Cells) The energy released in a spontaneous redox reaction can be used to perform electrical work △G=△H-T△S Voltaic or Galvanic cells are devices in which electron transfer occurs via an external circuit rather than directly between reactants. Switch 1.10 The actual charge on the Voltmeter electrode are zero. anode Na Cu Anode:the NO cathode electrode at Zn2+ which the NO Cathode:the electrode oxidation Zn(s)-Zn2i (ag)+2e Cu2 (ng)+2e Culs) at which the reduction occurs Movement of cations occurs Movement of anionsChap.20 Voltaic Cells (Galvanic Cells) The energy released in a spontaneous spontaneous redox reaction reaction can be used to perform electrical work. Voltaic or Galvanic cells are devices in which electron transfer occurs via an external circuit rather than directly between reactants. The actual charge on the ∆ = ∆ − ∆ G H T S Cathode: the electrode at which the reduction occurs Anode: the electrode at which the oxidation occurs The actual charge on the electrode are zero