正在加载图片...
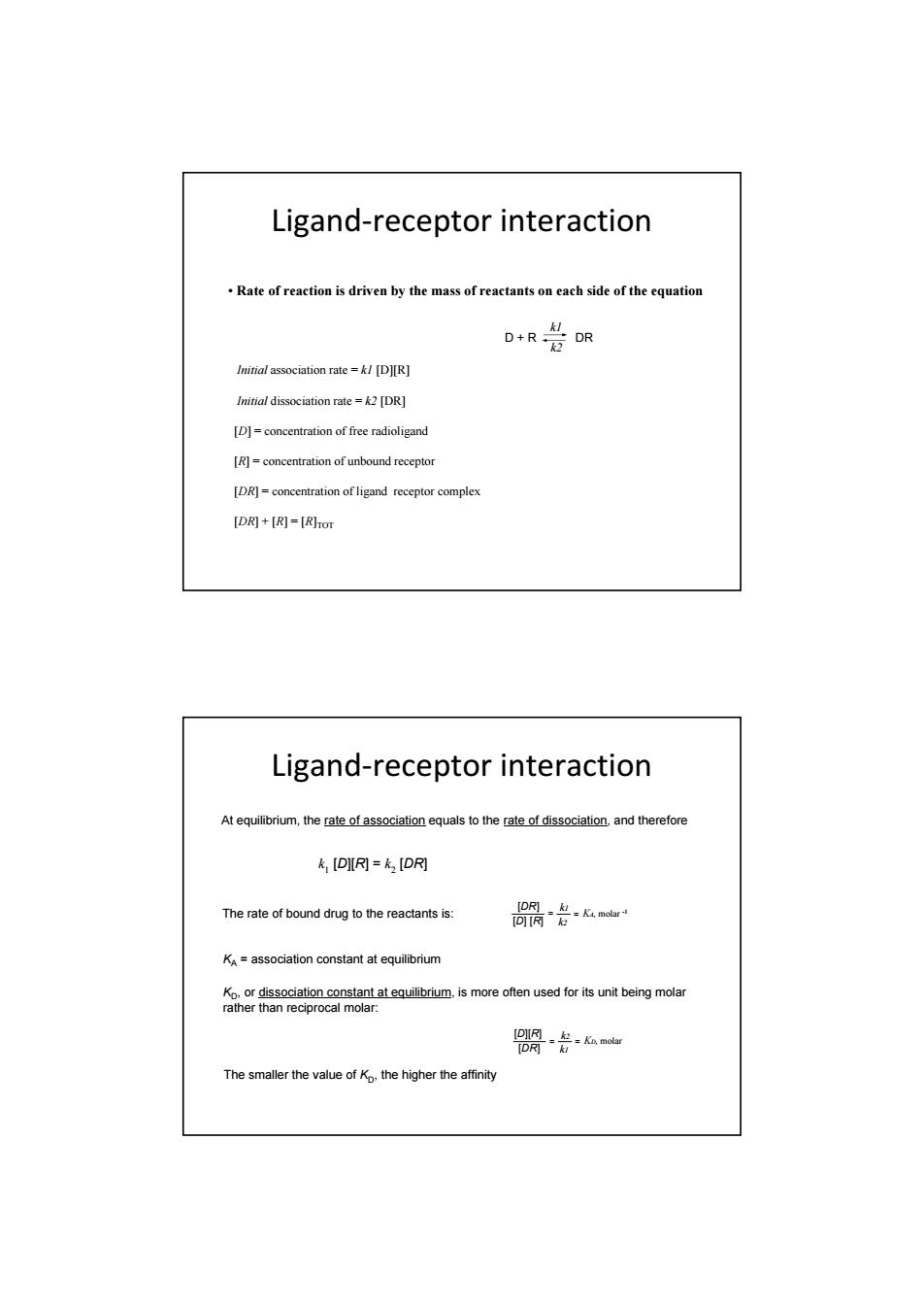
Ligand-receptor interaction Rate of reaction is driven by the mass of reactants on each side of the equation 0R岩R Initial association rate=kI[D][R] Initial dissociation rate=k2 [DR] [D]=concentration of free radioligand [R]=concentration of unbound receptor [DR]=concentration of ligand receptor complex [DR]+[R]=[RHTOT Ligand-receptor interaction At equilibrium,the rate of association equals to the rate of dissociation,and therefore k [DIIR]=k [DR] The rate of bound drug to the reactants is: [DR] -=molar 0阿 KA=association constant at equilibrium Kp.or dissociation constant at equilibrium,is more often used for its unit being molar rather than reciprocal molar: [DIg-是-Kmolar D风 The smaller the value of Kp.the higher the affinity• Rate of reaction is driven by the mass of reactants on each side of the equation Initial association rate = k1 [D][R] D + R k2 k1 DR Initial dissociation rate = k2 [DR] [D] = concentration of free radioligand [R] = concentration of unbound receptor [DR] = concentration of ligand receptor complex [DR] + [R] = [R]TOT Ligand‐receptor interaction At equilibrium, the rate of association equals to the rate of dissociation, and therefore k1 [D][R] = k2 [DR] The rate of bound drug to the reactants is: [D] [R] [DR] = = k1 k2 KA, molar -1 KA = association constant at equilibrium KD, or dissociation constant at equilibrium, is more often used for its unit being molar rather than reciprocal molar: [D][R] [DR] = = k2 k1 KD, molar The smaller the value of KD, the higher the affinity Ligand‐receptor interaction