正在加载图片...
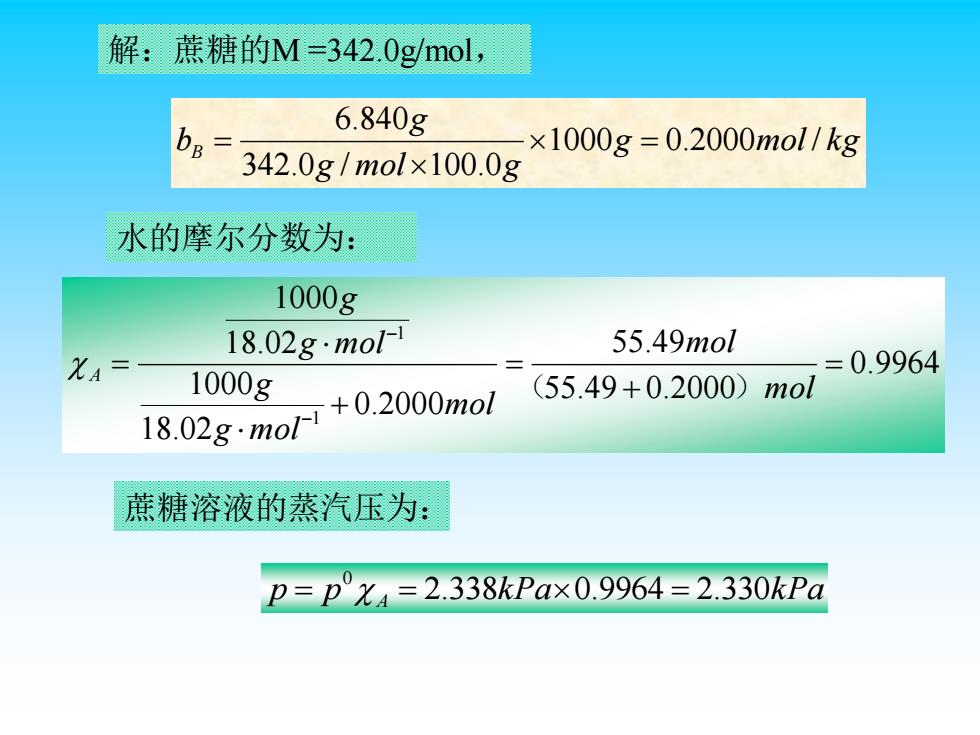
解:蔗糖的M=342.0g/mol, 6.840g bB= -×1000g=0.2000mol/kg 342.0g/mol×100.0 水的摩尔分数为: 1000g 18.02g.mol-1 55.49mol XA= =0.9964 1000g +0.2000mol (55.49+0.2000)mol 18.02gmo 蔗糖溶液的蒸汽压为: p=p°X4=2.338kPa×0.9964=2.330kPa 解:蔗糖的M =342.0g/mol, g mol k g g mol g g bB 1000 0.2000 / 342.0 / 100.0 6.840 = = 水的摩尔分数为: 0.9964 55.49 0.2000 55.49 0.2000 18.02 1000 18.02 1000 1 1 = + = + = − − mol mol mol g mol g g mol g A ( ) 蔗糖溶液的蒸汽压为: p p A 2.338kPa 0.9964 2.330kPa 0 = = =