正在加载图片...
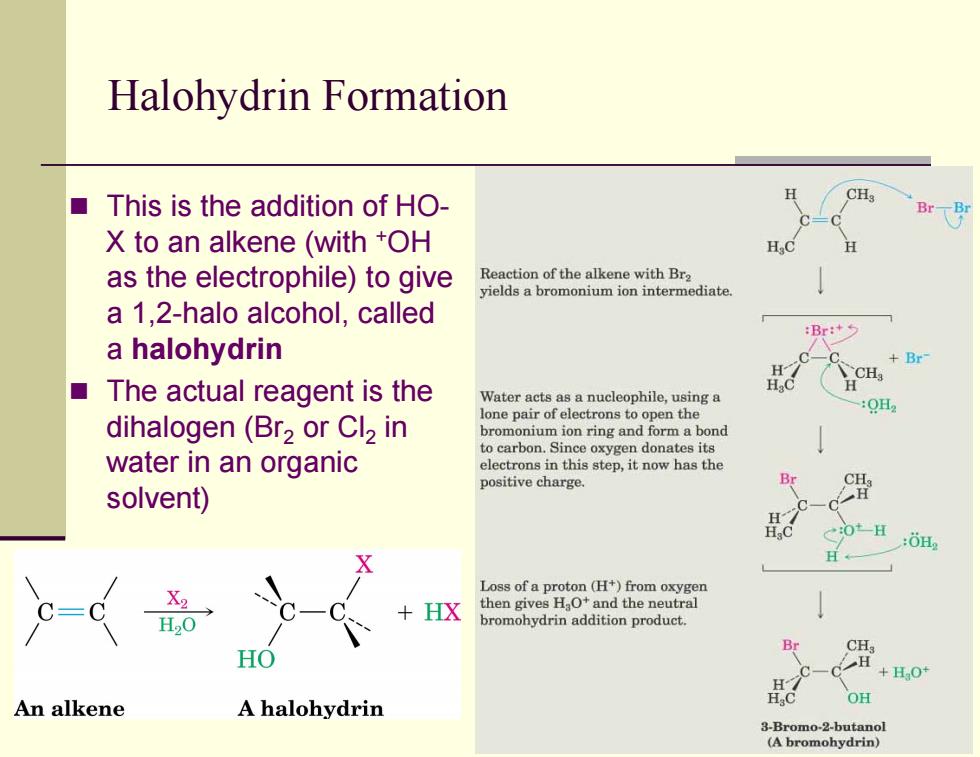
Halohydrin Formation This is the addition of HO- X to an alkene (with +OH as the electrophile)to give Reaction of the alkene with Br2 yields a bromonium ion intermediate. a 1,2-halo alcohol,called :Br:+ a halohydrin H CH The actual reagent is the .C Water acts as a nucleophile,using a lone pair of electrons to open the :0H2 dihalogen (Br2 or Cl2 in bromonium ion ring and form a bond to carbon.Since oxygen donates its water in an organic electrons in this step,it now has the positive charge. solvent) c0t-H :ǒH X Loss of a proton (H+)from oxygen X then gives HaO+and the neutral H.O HX bromohydrin addition product. Br CH HO +HO H An alkene A halohydrin OH 3-Bromo-2-butanol (A bromohydrin) Halohydrin Formation This is the addition of HOX to an alkene (with +OH as the electrophile) to give a 1,2-halo alcohol, called a halohydrin The actual reagent is the dihalogen (Br 2 or Cl 2 in water in an organic solvent)