正在加载图片...
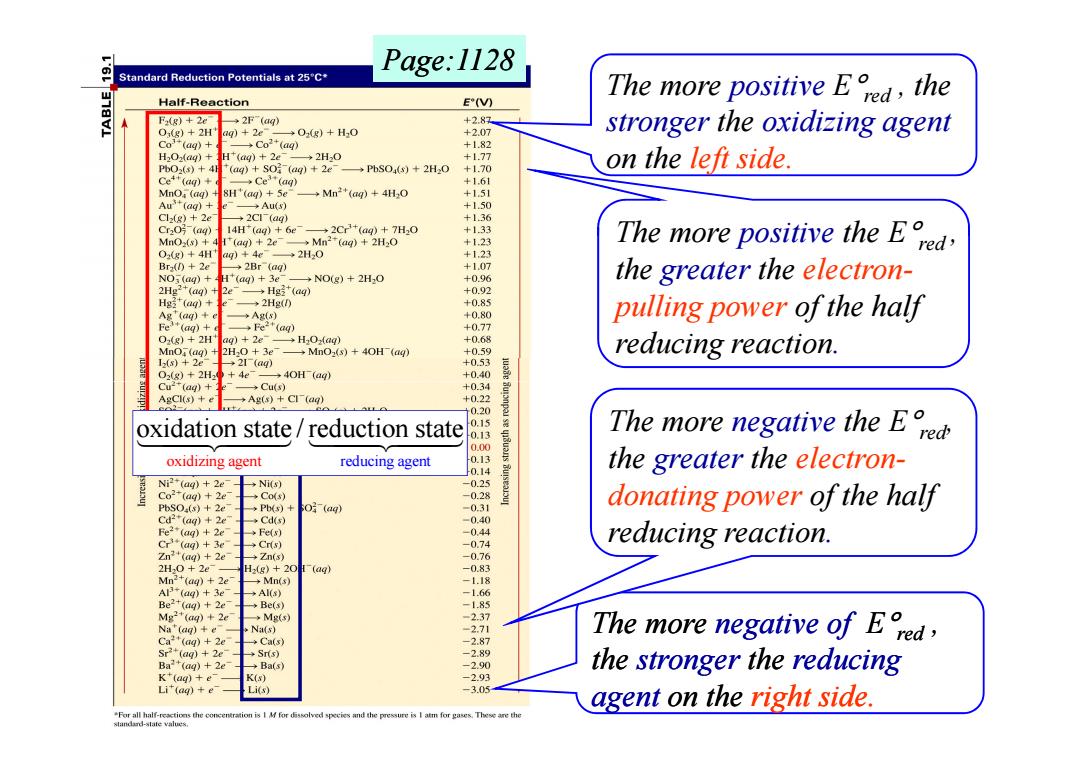
Page:1128 Standard Reduction Potentials at 25C* Half-Reaction E'(V) The more positive Eed the F2(g)+2e 2F(aq) +2.87 O,(g)+2H ag)+2e- O(g)+H.O +2.07 stronger the oxidizing agent Co(ag)+ →co2+(ag) +1.82 H202(ag)+H*(aq)+2e →2H0 +1.77 PbO2(s)+4H (aq)+SO (ag)+2e →P%S04()+2H0+1.70 on the left side. Ce+(ag)+ →Ce3+(ag) +1.61 MnO (aq) 8H'(ag)+5e →Mn2+(ag)+4H0 +1.51 Au(aq)+ Au(s) +1.50 Cl(g)+2e +2C1(ag) +136 CrzO月(ag) 14H(ag)+6e- →2Cr23+(aq)+7H20 +1.33 MnOx(s)+ H (ag)2e Mn2(aq)2H2O +1.23 The more positive the E O2(g)+4H ag)4e ◆2H20 +1.23 Bra()+2e →2Br(ag) +1.07 NO3(ag)+ H*(aq)3e →NO(g)+2H2O +0.96 the greater the electron- 2Hg2+(ag)+ 2 →Hg*(a +0.92 Hg(ag)+ →2Hg(0 +0.85 Ag"(ag)+ ◆Ag(s) +0.80 pulling power of the half Fe(aq)+ →Fe2t(ag) +0.77 O2(g)+2H" ag)2e- H2Oag) +0.68 MnO (ag)+2H2O 3e →MnO2(s)+4OH(ag) +059 reducing reaction. 12(s)+2e 21(q) +0.53 02(g)+2H2 +4e- 40H(aq) +0.40 Cu(ag)+ Cu(s) +0.34 AgCl(s)+ →Ags)+ClT(aq) +0.22 2= 0.20 oxidation state reduction state 0.15 0.13 The more negative the E 0.00 oxidizing agent reducing agent 0.13 0.14 the greater the electron- Ni2(aq)2e →Nis) -0.25 Co2(ag)2e →Co(s) -0.28 PbSOa(s)+2e >Pb(s)+ (aq) -0.31 donating power of the half Cd2*(aq)+2e →Cds) -0.40 Fe2(aq)2e Fe(s) -0.44 C3+(ag)+3e →Crts) -0.74 reducing reaction. Zn2(aq)+2e Zn(s) -0.76 2H0+2e H-(g)+20(aq) -0.83 Mn2+(aq)2e Mn(s) -1.18 Al+(aq)+3e →AI(s) -1.66 Be2(aq)+2e →Be(s) -1.85 Mg2"(aq)+2e →Mg() -2.37 Na (ag)+e Na(s) -2.71 Ca(ag)+2e The more negative of E, Ca(s) -2.87 Sr2*(aq)+2e →Srs) 2.89 Ba(aq)+2e →Ba(s) -2.90 the stronger the reducing K*(ag)+e K(s) -2.93 Li(aq)+e Li(s) -3.05 agent on the right side. "For all half-reactions the conc species and the pressure is I atm for gases.These are the standard-state values.The more positive E°red , the stronger the oxidizing agent on the left side. The more positive the E°red , the greater the electronpulling power of the half reducing reaction. Page:1128 The more negative of E°red , the stronger the reducing agent on the right side. The more negative the E°red, the greater the electrondonating power of the half reducing reaction. oxidizing agent reducing agent oxidation state / reduction state ����������