正在加载图片...
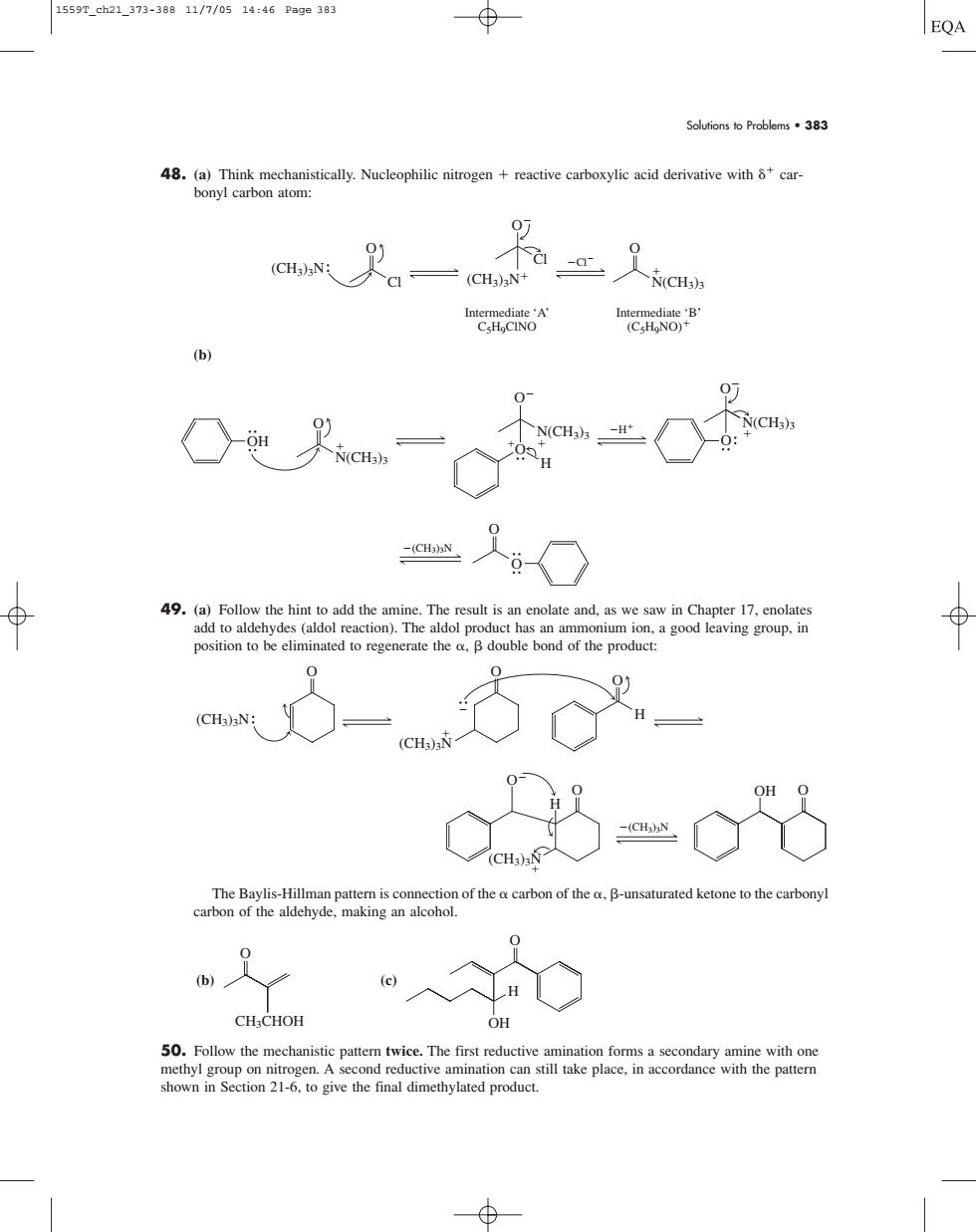
1559T_ch21_373-38811/7/0514:46Pa9e383 EQA Soutionso Problems33 48. acid derivative withcar (CH3)N CH 广(CH) 49.(a)Follow the hint to add the amine.The result is an enolate and,as we saw in Chapter 17,enolate add to aldehydes (aldol reaction).The aldol product has an ammonium ion.a good leaving group.in 03=c8 The bavlis-Hillman pattern is tion of the o carbon of the o B-unsaturated ketone to the carbony arbon of the aldehyde.making an alcohol CH-CHOE 50.Follow the mechanistic pattern twice.The first reductive amination for shown in Section 216.to give the final dimethylated product. Solutions to Problems • 383 48. (a) Think mechanistically. Nucleophilic nitrogen reactive carboxylic acid derivative with carbonyl carbon atom: (b) 49. (a) Follow the hint to add the amine. The result is an enolate and, as we saw in Chapter 17, enolates add to aldehydes (aldol reaction). The aldol product has an ammonium ion, a good leaving group, in position to be eliminated to regenerate the , double bond of the product: The Baylis-Hillman pattern is connection of the carbon of the , -unsaturated ketone to the carbonyl carbon of the aldehyde, making an alcohol. (b) (c) 50. Follow the mechanistic pattern twice. The first reductive amination forms a secondary amine with one methyl group on nitrogen. A second reductive amination can still take place, in accordance with the pattern shown in Section 21-6, to give the final dimethylated product. H OH O CH3CHOH O O (CH3)3N O O H OH (CH3)3N O O O (CH3)3N (CH3)3N H O O (CH3)3N H O N(CH3)3 OH O N(CH3)3 O H O N(CH3)3 O O Cl Cl (CH3)3N O Cl (CH3)3N O N(CH3)3 Intermediate ‘A’ C5H9ClNO Intermediate ‘B’ (C5H9NO) 1559T_ch21_373-388 11/7/05 14:46 Page 383����