正在加载图片...
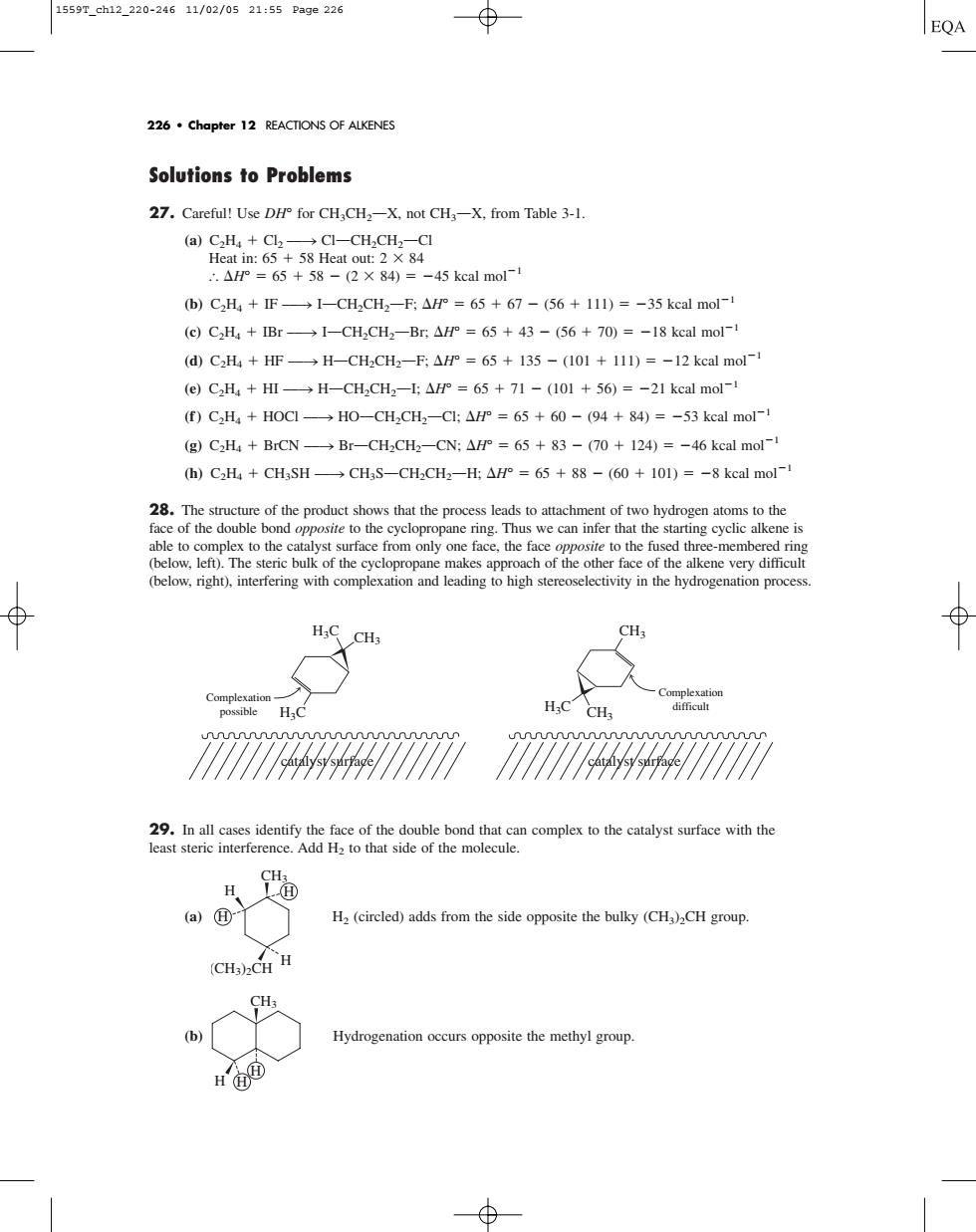
1559T_ch12_220-24611/02/0521:55Pa9e226 ⊕ EQA 226.Chapter 12 REACTIONS OF ALKENES Solutions to Problems 27.Careful!Use DHP for CH:CH-X.not CH-X.from Table 3-1. 45 kcal mol- b)CH4+F→1-CH,CH2-F:△P°=65+67-(56+11l)=-35 keal mol- (e)C2H IBr-I-CH2CH2-Br.AH=65+43-(56+70)=-18 kcal mol-1 (d)C2H+HF-H-CHCH2-F;AF =65 135-(101 111)=-12 keal mol- (e)CH4+H→H-CH,CH2-士△r°=65+71-(101+56)=-21 kcal mol-1 (f)C2H HOCI. HO-CH2CH2-Cl:AH =65+60-(94+84)=-53 kcal mol- (g)C2H+BrCN-Br-CHaCHa-CN:AF=65+83-(70+124)=-46 kcal mol-1 h)CH4+CHSH→CHS-CH,CH-H△H°=65+88-(60+10l)=-8 kcal mol ows that the process to complex o the caofemm (blow. othe ith d H.c HC CHs 29.In all cases identify the face of the double bond that can complex to the catalyst surface with the least steric interference.Add H to that side of the molecule. a)@ H (circled)adds from the side opposite the bulky (CH)CH group (CH3)CH H (b) Hydrogenation occurs opposite the methyl group. H的 Solutions to Problems 27. Careful! Use DH° for CH3CH2OX, not CH3OX, from Table 3-1. (a) C2H4 Cl2 88n ClOCH2CH2OCl Heat in: 65 58 Heat out: 2 84 H° 65 58 (2 84) 45 kcal mol1 (b) C2H4 IF 88n IOCH2CH2OF; H° 65 67 (56 111) 35 kcal mol1 (c) C2H4 IBr 88n IOCH2CH2OBr; H° 65 43 (56 70) 18 kcal mol1 (d) C2H4 HF 88n HOCH2CH2OF; H° 65 135 (101 111) 12 kcal mol1 (e) C2H4 HI 88n HOCH2CH2OI; H° 65 71 (101 56) 21 kcal mol1 (f ) C2H4 HOCl 88n HOOCH2CH2OCl; H° 65 60 (94 84) 53 kcal mol1 (g) C2H4 BrCN 88n BrOCH2CH2OCN; H° 65 83 (70 124) 46 kcal mol1 (h) C2H4 CH3SH 88n CH3SOCH2CH2OH; H° 65 88 (60 101) 8 kcal mol1 28. The structure of the product shows that the process leads to attachment of two hydrogen atoms to the face of the double bond opposite to the cyclopropane ring. Thus we can infer that the starting cyclic alkene is able to complex to the catalyst surface from only one face, the face opposite to the fused three-membered ring (below, left). The steric bulk of the cyclopropane makes approach of the other face of the alkene very difficult (below, right), interfering with complexation and leading to high stereoselectivity in the hydrogenation process. 29. In all cases identify the face of the double bond that can complex to the catalyst surface with the least steric interference. Add H2 to that side of the molecule. (a) H2 (circled) adds from the side opposite the bulky (CH3)2CH group. (b) Hydrogenation occurs opposite the methyl group. H CH3 H H H H CH3 (CH3)2CH H H CH3 H3C Complexation possible H3C catalyst surface catalyst surface CH3 CH3 H3C Complexation difficult 226 • Chapter 12 REACTIONS OF ALKENES 1559T_ch12_220-246 11/02/05 21:55 Page 226