正在加载图片...
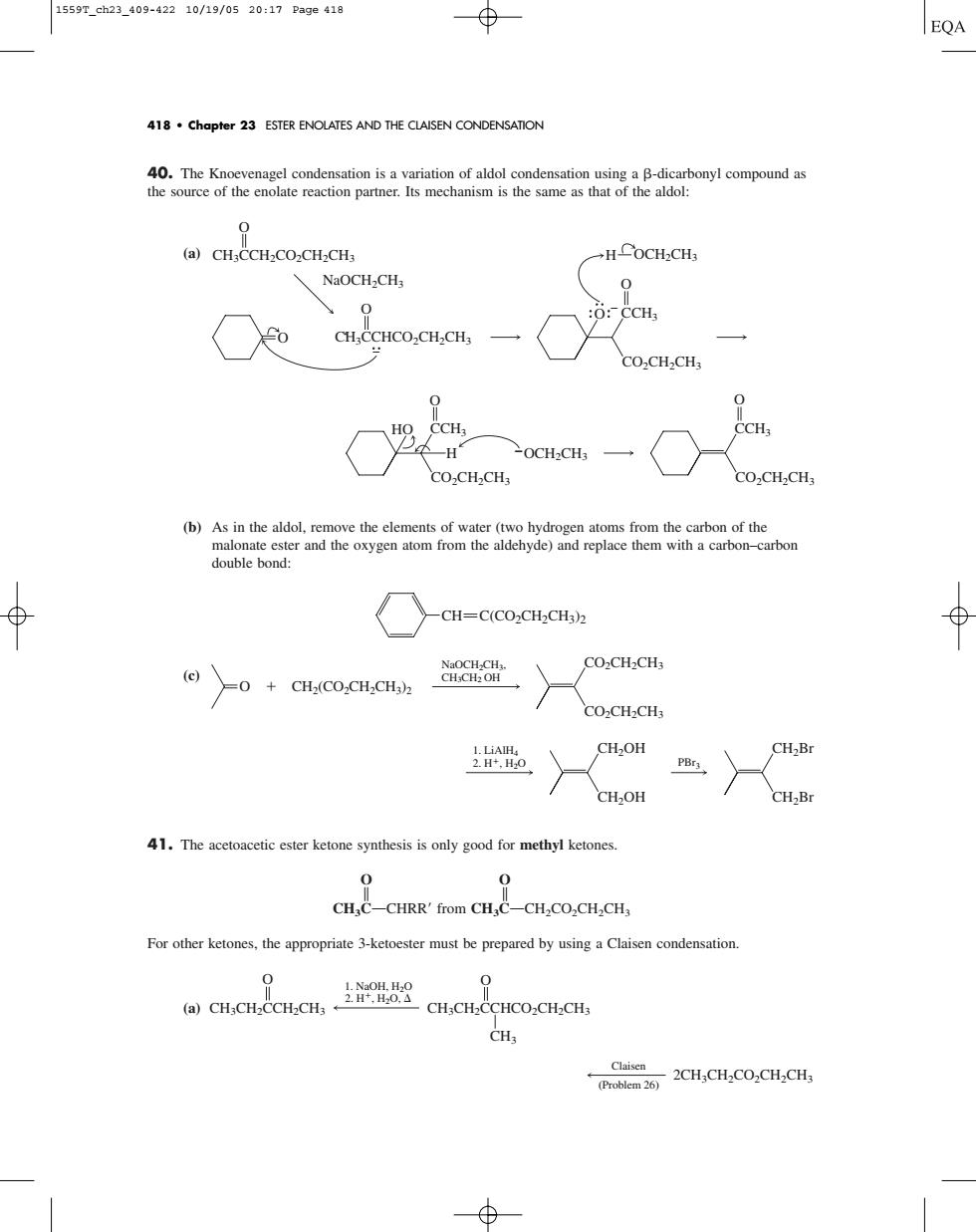
15597_ch23_409-42210/19/0520:17Page419 418.Chapter 23 ESTER ENOLATES AND THE CLAISEN CONDENSATION 40.The Kn usm is the same a (a)CH.CCH-CO2CH2CH .HCoCH.CH NaOCH2CH :o:CCH CH.CCHCO.CH.CH, CO.CH2CHs CH CH: OCH:CHs CO.CHCH CO2CH2CH3 (b) arbon double bond: -CH=C(CO2CH2CH3)2 &098 CO:CH2CH CO2CH2CH3 CH2OH CH,B 41.The acetoacetic ester ketone synthesis is only good for methyl ketones. CH,C-CHRR'from CH,C-CH2CO,CH2CH, For other ketones,the appropriate 3-ketoester must be prepared by using a Claisen condensation. 0 0 CHCO2CH.CHs CHa CH.CH.CO.CH.CH40. The Knoevenagel condensation is a variation of aldol condensation using a -dicarbonyl compound as the source of the enolate reaction partner. Its mechanism is the same as that of the aldol: (a) (b) As in the aldol, remove the elements of water (two hydrogen atoms from the carbon of the malonate ester and the oxygen atom from the aldehyde) and replace them with a carbon–carbon double bond: (c) 41. The acetoacetic ester ketone synthesis is only good for methyl ketones. O O B B CH3COCHRR from CH3COCH2CO2CH2CH3 For other ketones, the appropriate 3-ketoester must be prepared by using a Claisen condensation. (a) Claisen (Problem 26) 2CH3CH2CO2CH2CH3 1. NaOH, H2O 2. H, H2O, CH3CH2CCHCO2CH2CH3 CH3 O CH3CH2CCH2CH3 O 1. LiAlH4 2. H, H2O PBr3 CH2OH CH2OH CH2Br CH2Br O CH2(CO2CH2CH3) 2 NaOCH2CH3, CH3CH2 OH CO2CH2CH3 CO2CH2CH3 CH C(CO2CH2CH3)2 OCH2CH3 O CCH3 CO2CH2CH3 O CCH3 CO2CH2CH3 HO H NaOCH2CH3 CH3CCH2CO2CH2CH3 O CH3CCHCO2CH2CH3 O O O O CCH3 CO2CH2CH3 H OCH2CH3 418 • Chapter 23 ESTER ENOLATES AND THE CLAISEN CONDENSATION 1559T_ch23_409-422 10/19/05 20:17 Page 418����