正在加载图片...
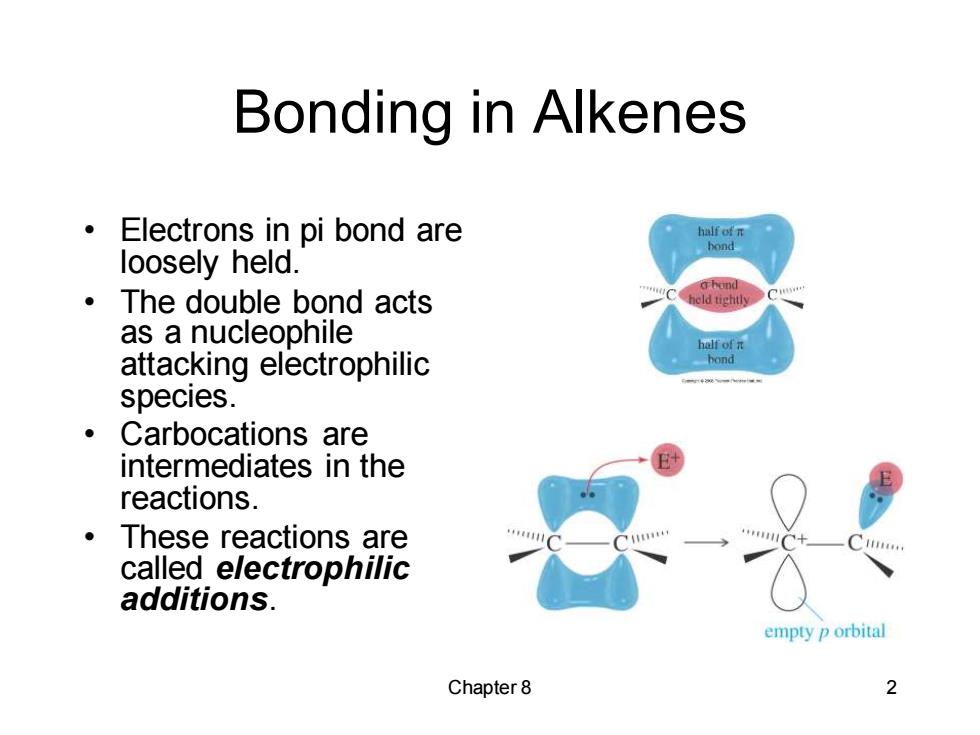
Bonding in Alkenes Electrons in pi bond are half of n loosely held. ·The double bond acts as a nucleophile hof元 attacking electrophilic hond species. ·Carbocations are intermediates in the reactions. ·These reactions are called electrophilic additions. empty p orbital Chapter 8 2Chapter 8 2 Bonding in Alkenes • Electrons in pi bond are loosely held. • The double bond acts as a nucleophile attacking electrophilic species. • Carbocations are intermediates in the reactions. • These reactions are called electrophilic additions