正在加载图片...
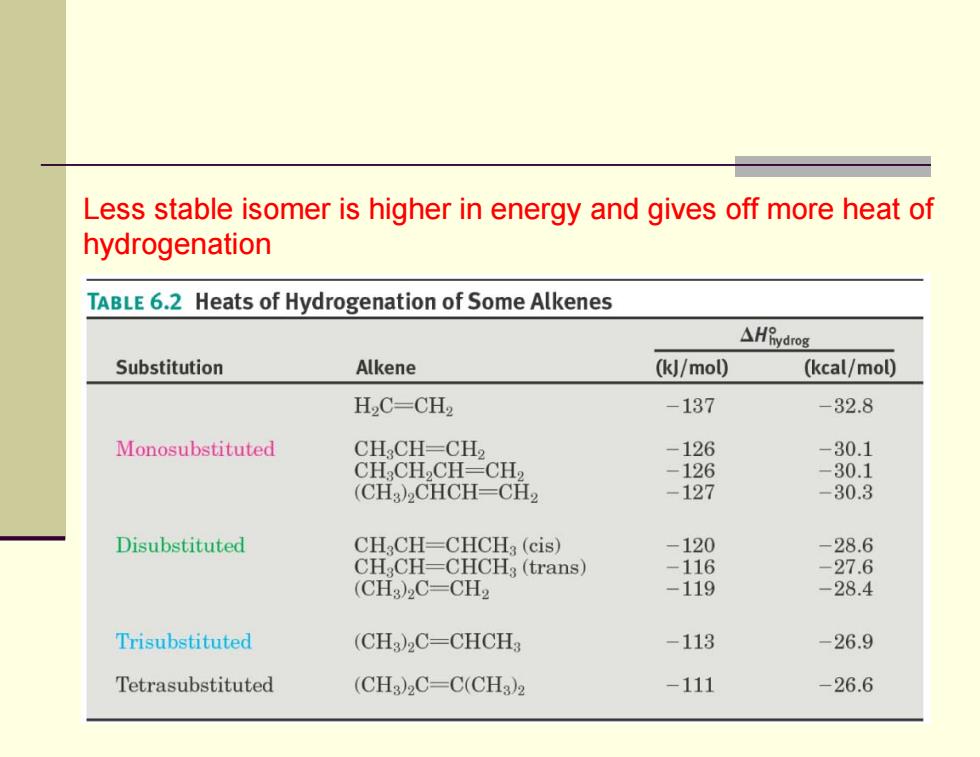
Less stable isomer is higher in energy and gives off more heat of hydrogenation TABLE 6.2 Heats of Hydrogenation of Some Alkenes △HBydrog Substitution Alkene (kJ/mol) (kcal/mol) H.C-CH2 -137 32.8 Monosubstituted CH CH-CH2 -126 -30.1 CH:CH,CH=CH2 -126 -30.1 (CHa)2CHCH=CH2 -127 -30.3 Disubstituted CH2CH-CHCHa(cis) -120 -28.6 CH:CH=CHCH:(trans) -116 -27.6 (CH)2C-CH2 -119 -28.4 Trisubstituted (CHa)2C-CHCH -113 -26.9 Tetrasubstituted (CH3)2C=C(CH3)2 -111 26.6Less stable isomer is higher in energy and gives off more heat of hydrogenation