正在加载图片...
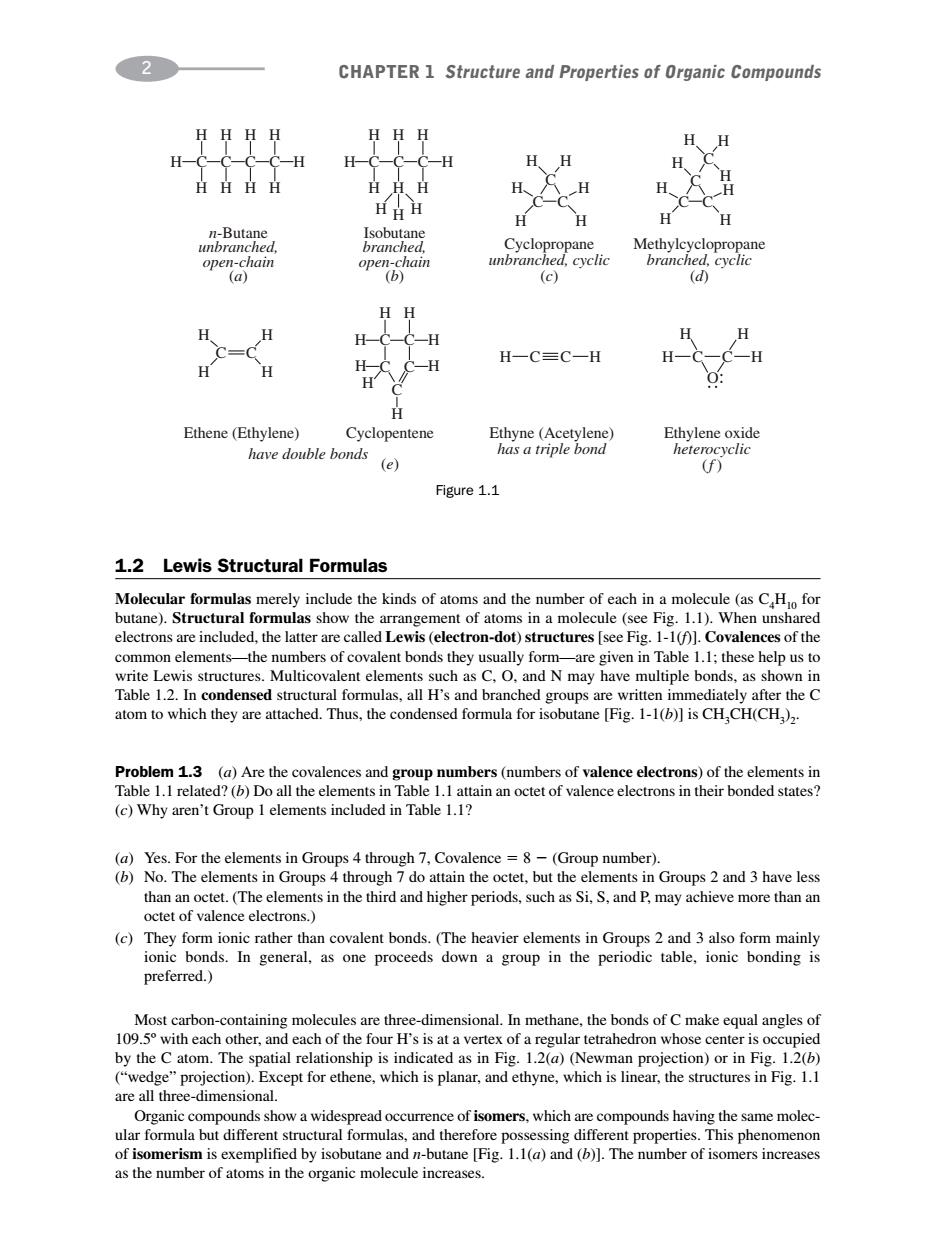
2● CHAPTER 1 Structure and Properties of Organic Compounds HHHH HH H-C-C-C-C-H H- HH H HHHH H H H H n8c (c) (d) HH Hc-H H H-C=C-H H H Ethene(Ethylene) Cyclopentene have double bonds (e) Figure 1.1 1.2 Lewis Structural Formulas Molecular formulas merely include the kinds of atom and the each in )St e number of electrons are included.the latter are called Lewis (electron-dot)structures see Fig.Covalences of the common elements-the numbers of covalent bonds they usually form-are given in Table 1.1:these help us to wis structures.Multicovalent clements such a C.O.and N may have multiple bonds,as shown in om to dens they are atta CH.CH(CH . Problem 1.3 (a)Are the covalences and group numbers(numbers of valence electrons)of the elements in 1.1 relate ()Why aren Grup lements incluhrone d in (a)Yes.For the elements in Groups 4 through 7,Covalence=8-(Group number). (b)No.The elements in Groups 4 through 7 do attain the octet,but the elements in Groups 2 and 3 have less octet of ence clectrons.d andr enods.suandmay acheverea (c)They fom dson c rather than covalent bond Most c th by the C atom.The spatial relationship is indicated as in Fig.1.2(a)(Newman projection)or in Fig.1.2(b) xcept for ethene,which is planar,and ethyne,which is linear,the structures in Fig.1.1 are ulas ur s Thi of isomerism is exemplified by isobutane and n-butane Fig1.1(a)and (The number of isomers increases as the number of atoms in the organic molecule increases. 1.2 Lewis Structural Formulas Molecular formulas merely include the kinds of atoms and the number of each in a molecule (as C4H10 for butane). Structural formulas show the arrangement of atoms in a molecule (see Fig. 1.1). When unshared electrons are included, the latter are called Lewis (electron-dot) structures [see Fig. 1-1(f)]. Covalences of the common elements—the numbers of covalent bonds they usually form—are given in Table 1.1; these help us to write Lewis structures. Multicovalent elements such as C, O, and N may have multiple bonds, as shown in Table 1.2. In condensed structural formulas, all H’s and branched groups are written immediately after the C atom to which they are attached. Thus, the condensed formula for isobutane [Fig. 1-1(b)] is CH3CH(CH3)2. Problem 1.3 (a) Are the covalences and group numbers (numbers of valence electrons) of the elements in Table 1.1 related? (b) Do all the elements in Table 1.1 attain an octet of valence electrons in their bonded states? (c) Why aren’t Group 1 elements included in Table 1.1? (a) Yes. For the elements in Groups 4 through 7, Covalence 8 (Group number). (b) No. The elements in Groups 4 through 7 do attain the octet, but the elements in Groups 2 and 3 have less than an octet. (The elements in the third and higher periods, such as Si, S, and P, may achieve more than an octet of valence electrons.) (c) They form ionic rather than covalent bonds. (The heavier elements in Groups 2 and 3 also form mainly ionic bonds. In general, as one proceeds down a group in the periodic table, ionic bonding is preferred.) Most carbon-containing molecules are three-dimensional. In methane, the bonds of C make equal angles of 109.5º with each other, and each of the four H’s is at a vertex of a regular tetrahedron whose center is occupied by the C atom. The spatial relationship is indicated as in Fig. 1.2(a) (Newman projection) or in Fig. 1.2(b) (“wedge” projection). Except for ethene, which is planar, and ethyne, which is linear, the structures in Fig. 1.1 are all three-dimensional. Organic compounds show a widespread occurrence of isomers, which are compounds having the same molecular formula but different structural formulas, and therefore possessing different properties. This phenomenon of isomerism is exemplified by isobutane and n-butane [Fig. 1.1(a) and (b)]. The number of isomers increases as the number of atoms in the organic molecule increases. 2 CHAPTER 1 Structure and Properties of Organic Compounds H C C H H CC C C H H HH O H H H H n-Butane unbranched, open-chain (a) Isobutane branched, open-chain (b) Cyclopropane unbranched, cyclic (c) Methylcyclopropane branched, cyclic (d) Ethene (Ethylene) have double bonds Cyclopentene (e) Ethyne (Acetylene) has a triple bond Ethylene oxide heterocyclic (f ) H H H C H H C H H C H H C H H H C C H C C C H H H H H H H H C H H H H H C H H H H H HH H C C H H H H H H H C C C C C C H Figure 1.1��