正在加载图片...
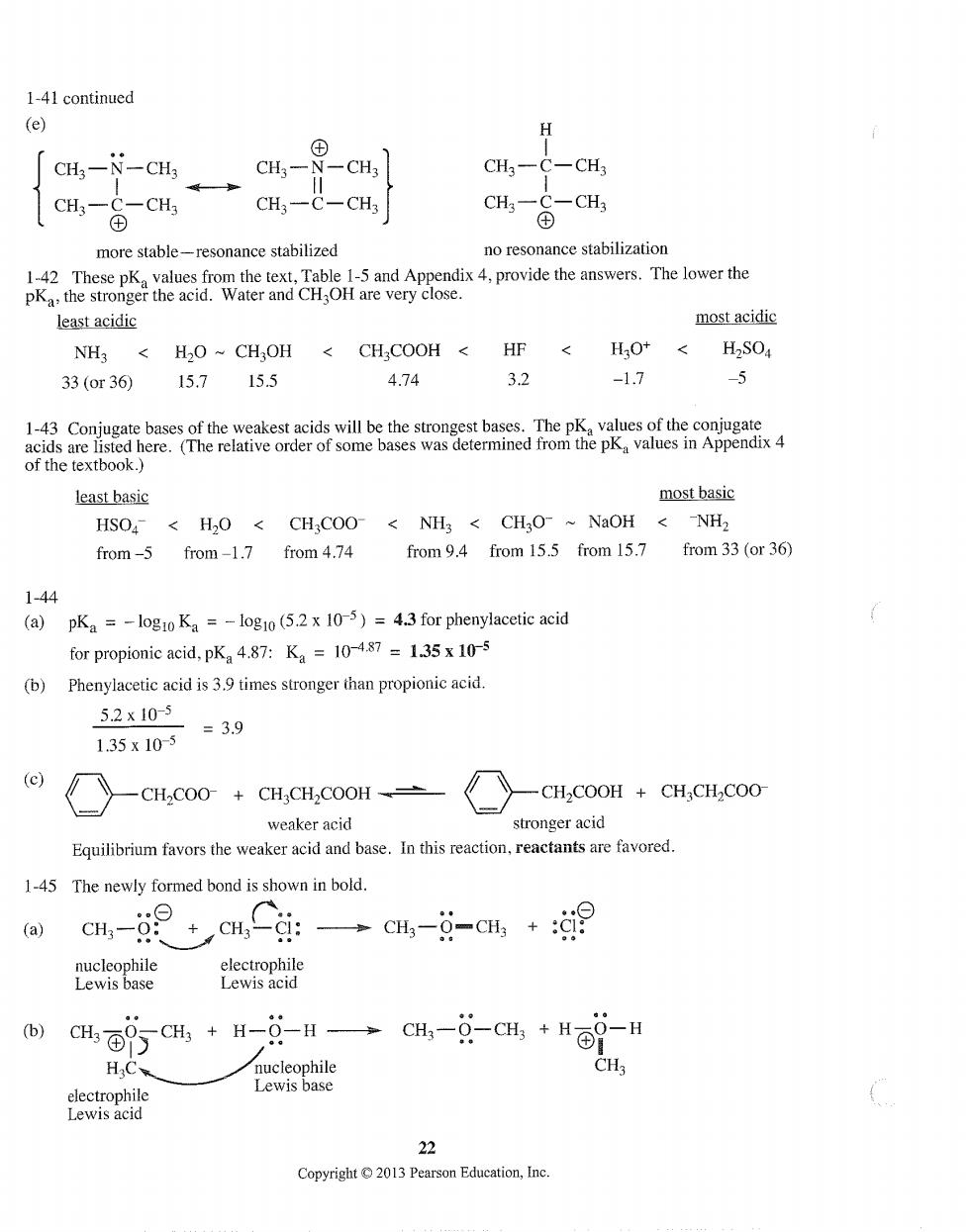
1-41 continued (e) CHa-N-CH3 CH3一N -CH3 CH一C-CH CH--CH CH3-C 一CH CH一 -CH3 more stable-resonance stabilized no resonance stabilization 1-42 These pK values from the text,Table 1-5 and Appendix 4,provide the answers.The lower the the stronge the aid.Water and CH are verycos least acidic most acidic NH< CHCOOH HF < H30+<H,SO4 33(or36 15.7 155 4.74 3.2 -1.7 -5 出出两 least basic most basic HSO<H2O<CH;COO<NH3 CH;O-~NaOH <NH2 from-5 from-1.7 from 4.74 from9.4 from 15.5 from 15.7 from 33(or 36) 1-44 (a) pKa =-logio Ka =-log10(5.2 x 10-5)=4.3 for phenylacetic acid for propionic acid,pKa 4.87:Ka=10-487=1.35x10-5 (b) Phenylacetic acid is39 times stronger than propionic acid. 5.2x10-5 =3.9 1.35x10-5 (c) —CH,C00+CH,CH,COOH± -CH,COOH CHCHCOO weaker acid stronger acid Equilibrium favors the weaker acid and base.In this reaction,reactants are favored. 1-45 The newly formed bond is shown in bold (a) c-g cn.Cci: →c4--c4,+9 nucleophile electrophile Lewis base Lewis acid (b) HC nucleophile Lewis base 22 Copyright2013 Pearson Education,Inc