正在加载图片...
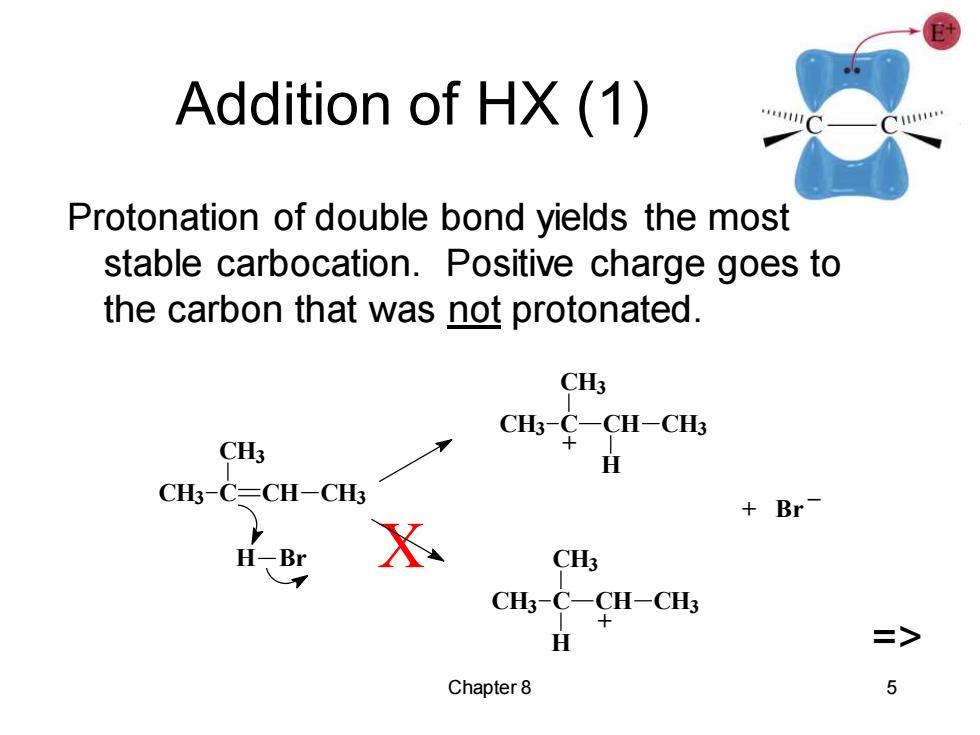
Addition of HX(1) Protonation of double bond yields the most stable carbocation.Positive charge goes to the carbon that was not protonated. CH3 CH3 CHs¥CH-C4 H CH3-C=CH-CH3 Br H-Br X CH3 CH3-C-CH-CH3 H => Chapter 8 5Chapter 8 5 Addition of HX (1) Protonation of double bond yields the most stable carbocation. Positive charge goes to the carbon that was not protonated. X => + Br _ + + CH3 C CH3 CH CH3 H CH3 C CH3 CH CH3 H H Br CH3 C CH3 CH CH3