正在加载图片...
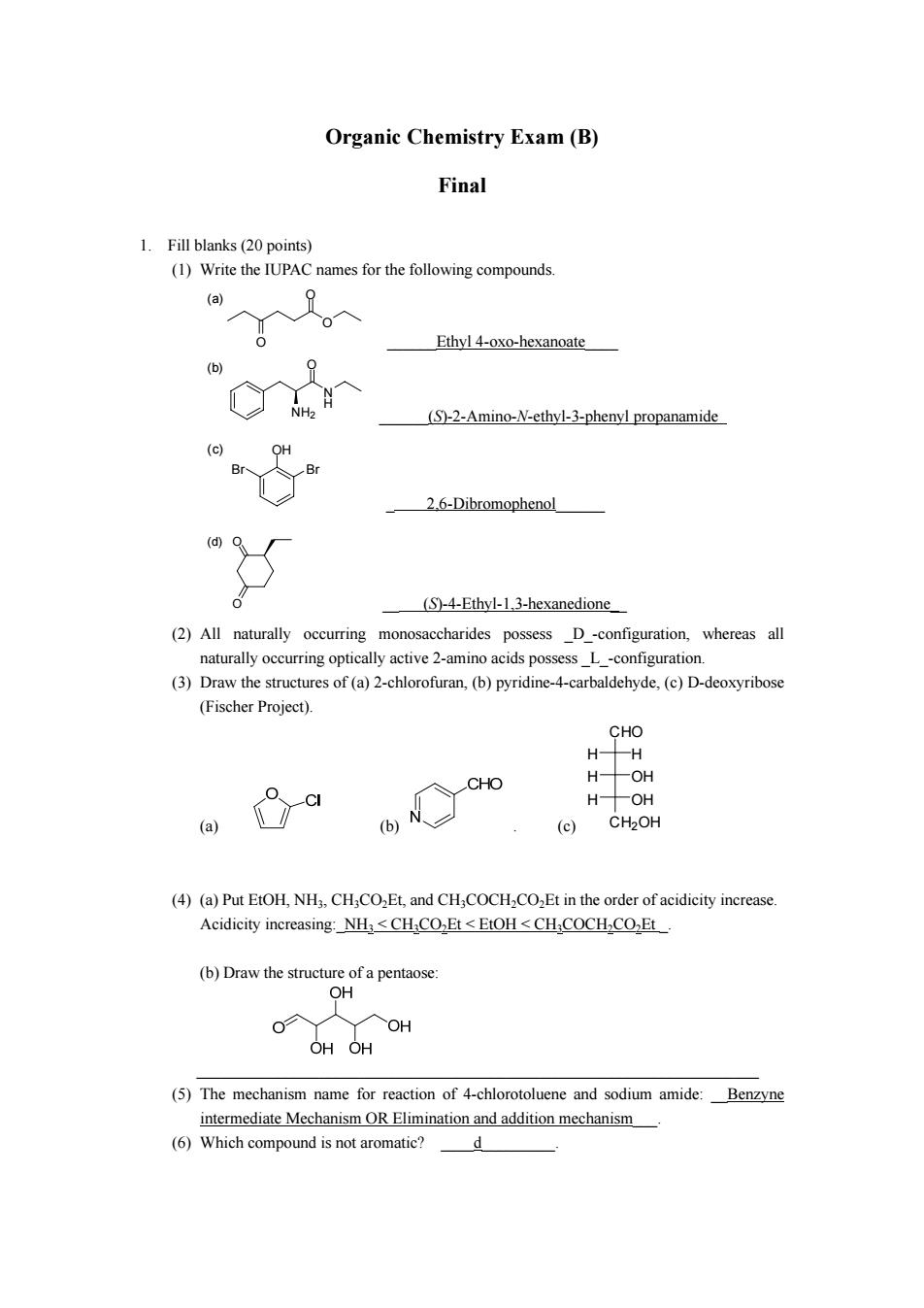
Organic Chemistry Exam(B) Final 1.Fill blanks(20 points) (1)Write the IUPAC names for the following compounds. (a) Ethyl 4-oxo-hexanoate (b) (S)-2-Amino-N-ethyl-3-phenyl propanamide 2.6-Dibromophenol (S)-4-Ethyl-1,3-hexanedione (2)All naturally occurring monosaccharides possess D-configuration,whereas all naturally occurring optically active 2-amino acids possess L -configuration. (3)Draw the structures of (a)2-hlorofuran,(b)pyridine-4-carbaldehyde.()D-deoxyribose (Fischer Project) CHO H-H H-OH a H-OH (c)CH2OH (4)(a)Put EtOH,NH3.CHCOEt,and CH,COCHCOEt in the order of acidicity increase Acidicity increasing:NH3<CH COEt <EtOH<CH COCH-COzEt OHOH (5)The mechanism name for reaction of 4-chlorotoluene and sodium amide:Benzyne intermediate Mechanism OR Elimination and addition mechanism (6)Which compound is not aromatic? d Organic Chemistry Exam (B) Final 1. Fill blanks (20 points) (1) Write the IUPAC names for the following compounds. O O (a) O ______Ethyl 4-oxo-hexanoate____ N H NH2 (b) O ______(S)-2-Amino-N-ethyl-3-phenyl propanamide OH Br Br (c) _ 2,6-Dibromophenol______ O O (d) __ (S)-4-Ethyl-1,3-hexanedione__ (2) All naturally occurring monosaccharides possess _D_-configuration, whereas all naturally occurring optically active 2-amino acids possess _L_-configuration. (3) Draw the structures of (a) 2-chlorofuran, (b) pyridine-4-carbaldehyde, (c) D-deoxyribose (Fischer Project). (a) (b) . (c) H H CHO H OH H OH CH2OH (4) (a) Put EtOH, NH3, CH3CO2Et, and CH3COCH2CO2Et in the order of acidicity increase. Acidicity increasing:_NH3 < CH3CO2Et < EtOH < CH3COCH2CO2Et _. (b) Draw the structure of a pentaose: _____________________________________________________________________ (5) The mechanism name for reaction of 4-chlorotoluene and sodium amide: __Benzyne intermediate Mechanism OR Elimination and addition mechanism___. (6) Which compound is not aromatic? ____d_________