正在加载图片...
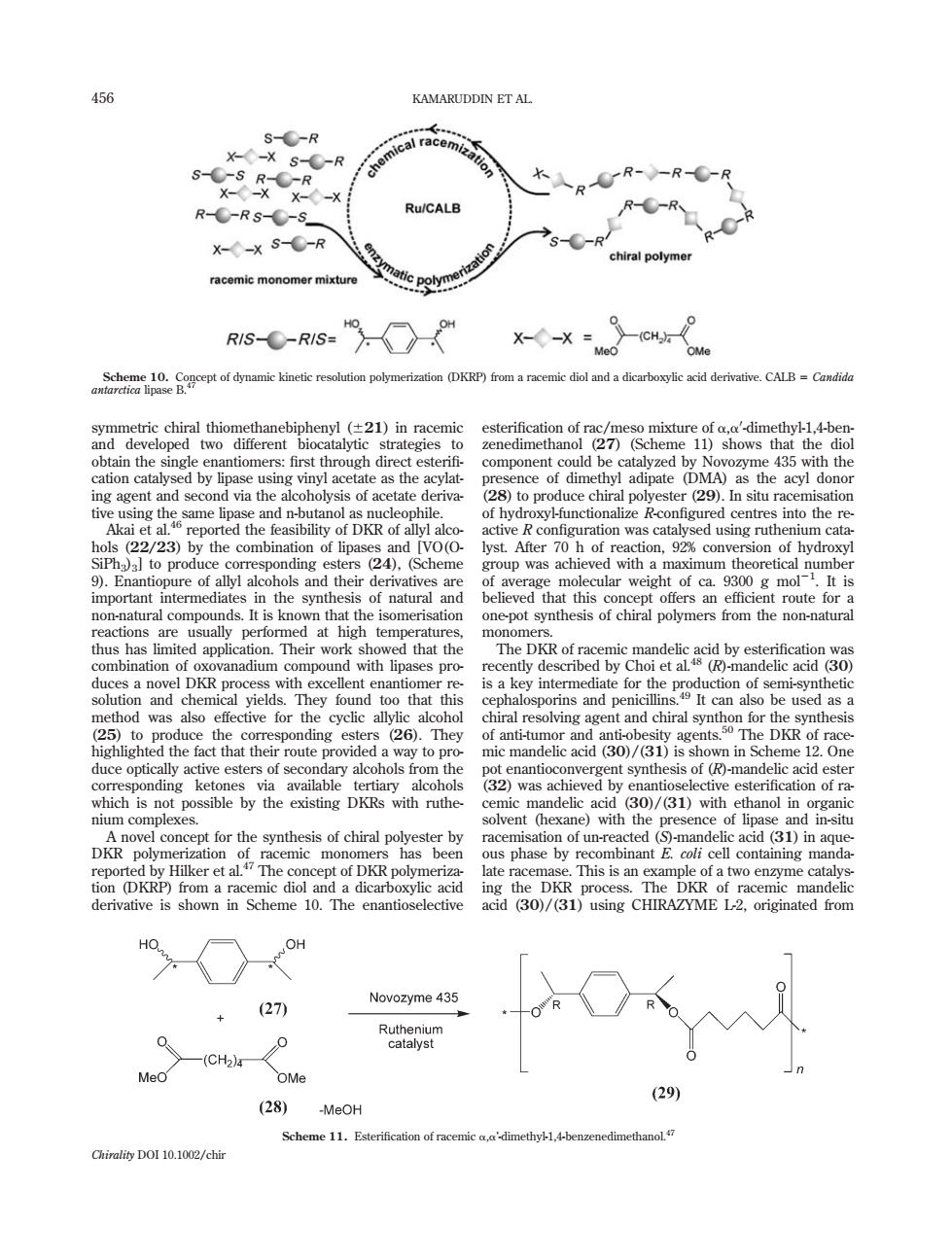
456 KAMARUDDIN ET AL al racem S-C-R pR--R-C-R R--RS-C-s Ru/CALB R--R chiral polymer racemic monomer mixture RIS--RIS X--x= fymi kinee e oyeri KRP)m diol andioevCAl obtain the single enantiomers:first through dire component could b e 435 with the 00 lyst.After 7 ction,92%conv )was a actions are usua at high tem the rec method was as efective for the cyclic allylic alcohol mic mandelic acid (D is shown in Scheme 12on cdocepiagarcteeetcrsafsoonihp'atlcohos om the sation of un-rea cted (5)-mandelic acid (3D)in ag acemic mon DKR originat HO OH Novozyme 435 + (27) Rtegm -(CH2)- OMe (29) (28) -MeOH Scheme 11.Esterification of 1,4-benzenedimethano Chirality DO110.1002/chir symmetric chiral thiomethanebiphenyl (621) in racemic and developed two different biocatalytic strategies to obtain the single enantiomers: first through direct esterifi- cation catalysed by lipase using vinyl acetate as the acylating agent and second via the alcoholysis of acetate derivative using the same lipase and n-butanol as nucleophile. Akai et al.46 reported the feasibility of DKR of allyl alcohols (22/23) by the combination of lipases and [VO(OSiPh3)3] to produce corresponding esters (24), (Scheme 9). Enantiopure of allyl alcohols and their derivatives are important intermediates in the synthesis of natural and non-natural compounds. It is known that the isomerisation reactions are usually performed at high temperatures, thus has limited application. Their work showed that the combination of oxovanadium compound with lipases produces a novel DKR process with excellent enantiomer resolution and chemical yields. They found too that this method was also effective for the cyclic allylic alcohol (25) to produce the corresponding esters (26). They highlighted the fact that their route provided a way to produce optically active esters of secondary alcohols from the corresponding ketones via available tertiary alcohols which is not possible by the existing DKRs with ruthenium complexes. A novel concept for the synthesis of chiral polyester by DKR polymerization of racemic monomers has been reported by Hilker et al.47 The concept of DKR polymerization (DKRP) from a racemic diol and a dicarboxylic acid derivative is shown in Scheme 10. The enantioselective esterification of rac/meso mixture of a,a0 -dimethyl-1,4-benzenedimethanol (27) (Scheme 11) shows that the diol component could be catalyzed by Novozyme 435 with the presence of dimethyl adipate (DMA) as the acyl donor (28) to produce chiral polyester (29). In situ racemisation of hydroxyl-functionalize R-configured centres into the reactive R configuration was catalysed using ruthenium catalyst. After 70 h of reaction, 92% conversion of hydroxyl group was achieved with a maximum theoretical number of average molecular weight of ca. 9300 g mol21 . It is believed that this concept offers an efficient route for a one-pot synthesis of chiral polymers from the non-natural monomers. The DKR of racemic mandelic acid by esterification was recently described by Choi et al.48 (R)-mandelic acid (30) is a key intermediate for the production of semi-synthetic cephalosporins and penicillins.49 It can also be used as a chiral resolving agent and chiral synthon for the synthesis of anti-tumor and anti-obesity agents.50 The DKR of racemic mandelic acid (30)/(31) is shown in Scheme 12. One pot enantioconvergent synthesis of (R)-mandelic acid ester (32) was achieved by enantioselective esterification of racemic mandelic acid (30)/(31) with ethanol in organic solvent (hexane) with the presence of lipase and in-situ racemisation of un-reacted (S)-mandelic acid (31) in aqueous phase by recombinant E. coli cell containing mandalate racemase. This is an example of a two enzyme catalysing the DKR process. The DKR of racemic mandelic acid (30)/(31) using CHIRAZYME L-2, originated from Scheme 10. Concept of dynamic kinetic resolution polymerization (DKRP) from a racemic diol and a dicarboxylic acid derivative. CALB 5 Candida antarctica lipase B.47 Scheme 11. Esterification of racemic a,a’-dimethyl-1,4-benzenedimethanol.47 456 KAMARUDDIN ET AL. Chirality DOI 10.1002/chir