正在加载图片...
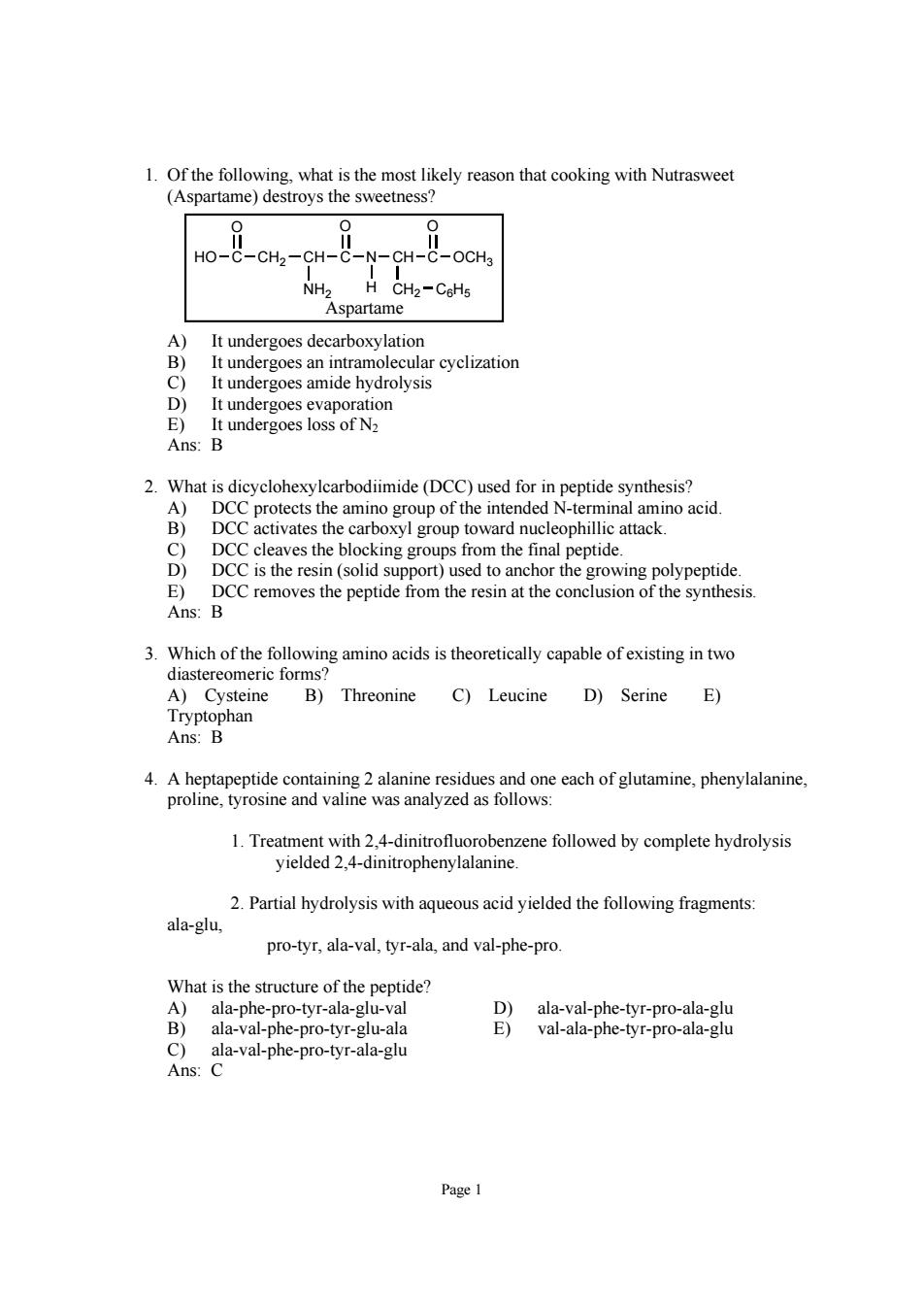
1.Of the following.what is the most likely reason that cooking with Nutrasweet (Aspartame)destroys the sweetness? HO-C-CH2-CH-C CH-C-OCHa NH2 CHz-CeHs Aspartame It undergoes decarboxylatior t un ergoes an intramol rcyclization D ergoes amide E)It undergoes loss of N Ans:B 2.What is dicyclohexylearbodiimide(DCC)used for in peptide synthesis? DCC protects the oxyl group towar eophill es the n the id ng gro ntia E)DCC nthesis Ans:B 3.Which of the following amino acids is theoretically capable of existing in two diastereomeric forms A)Cysteine B)Threonine C)Leucine D)Serine E) 4.A heptapeptide containing 2 alanine residues and one each of glutamine,phenylalanine, proline,tyrosine and valine was analyzed as follows: 1.Treatment with 2,4-dinitrofluorobenzene followed by complete hydrolysis yielded 2.4-dinitrophenylalanine. 2.Partial hydrolysis with aqueousacid yielded the following fragments ala-glu pro-tyr,ala-val,tyr-ala,and val-phe-pro What is the structure of the peptide? D)ala-val-phe-tyr-pro-ala-glu ala-val-phe-pro-tyr-glu-ala val-ala-phe-tyr-pro-ala-gluPage 1 1. Of the following, what is the most likely reason that cooking with Nutrasweet (Aspartame) destroys the sweetness? HO C O CH2 CH NH2 C O N CH H CH2 C6H5 C O OCH3 Aspartame A) It undergoes decarboxylation B) It undergoes an intramolecular cyclization C) It undergoes amide hydrolysis D) It undergoes evaporation E) It undergoes loss of N2 Ans: B 2. What is dicyclohexylcarbodiimide (DCC) used for in peptide synthesis? A) DCC protects the amino group of the intended N-terminal amino acid. B) DCC activates the carboxyl group toward nucleophillic attack. C) DCC cleaves the blocking groups from the final peptide. D) DCC is the resin (solid support) used to anchor the growing polypeptide. E) DCC removes the peptide from the resin at the conclusion of the synthesis. Ans: B 3. Which of the following amino acids is theoretically capable of existing in two diastereomeric forms? A) Cysteine B) Threonine C) Leucine D) Serine E) Tryptophan Ans: B 4. A heptapeptide containing 2 alanine residues and one each of glutamine, phenylalanine, proline, tyrosine and valine was analyzed as follows: 1. Treatment with 2,4-dinitrofluorobenzene followed by complete hydrolysis yielded 2,4-dinitrophenylalanine. 2. Partial hydrolysis with aqueous acid yielded the following fragments: ala-glu, pro-tyr, ala-val, tyr-ala, and val-phe-pro. What is the structure of the peptide? A) ala-phe-pro-tyr-ala-glu-val D) ala-val-phe-tyr-pro-ala-glu B) ala-val-phe-pro-tyr-glu-ala E) val-ala-phe-tyr-pro-ala-glu C) ala-val-phe-pro-tyr-ala-glu Ans: C