正在加载图片...
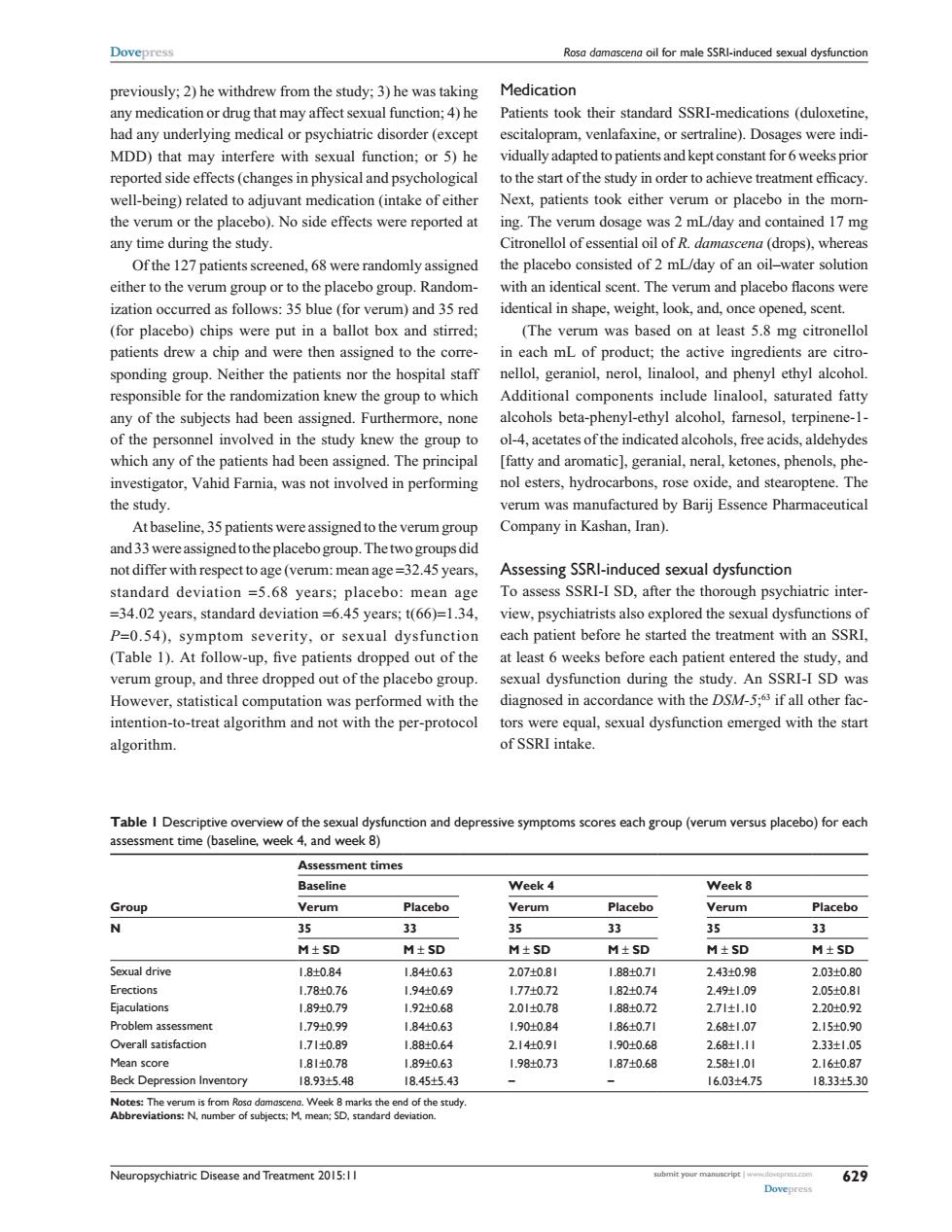
Dovepress Rosa damascena oil for male SSRl-induced sexual dysfunction previously;2)he withdrew from the study;3)he was taking Medication any medication or drug that may affect sexual function;4)he Patients took their standard SSRI-medications (duloxetine. had any underlying medical or psychiatric disorder(except escitalopram,venlafaxine,or sertraline).Dosages were indi- MDD)that may interfere with sexual function;or 5)he vidually adapted to patients and kept constant for 6 weeks prior reported side effects(changes in physical and psychological to the start of the study in order to achieve treatment efficacy. well-being)related to adjuvant medication(intake of either Next,patients took either verum or placebo in the morn- the verum or the placebo).No side effects were reported at ing.The verum dosage was 2 mL/day and contained 17 mg any time during the study. Citronellol of essential oil of R.damascena(drops),whereas Ofthe 127 patients screened,68 were randomly assigned the placebo consisted of 2 mL/day of an oil-water solution either to the verum group or to the placebo group.Random-with an identical scent.The verum and placebo flacons were ization occurred as follows:35 blue(for verum)and 35 red identical in shape,weight,look,and,once opened,scent. (for placebo)chips were put in a ballot box and stirred; (The verum was based on at least 5.8 mg citronellol patients drew a chip and were then assigned to the corre- in each mL of product;the active ingredients are citro- sponding group.Neither the patients nor the hospital staff nellol,geraniol,nerol,linalool,and phenyl ethyl alcohol. responsible for the randomization knew the group to which Additional components include linalool,saturated fatty any of the subjects had been assigned.Furthermore,none alcohols beta-phenyl-ethyl alcohol,farnesol,terpinene-1- of the personnel involved in the study knew the group to ol-4,acetates of the indicated alcohols,free acids,aldehydes which any of the patients had been assigned.The principal [fatty and aromatic],geranial,neral,ketones,phenols,phe- investigator,Vahid Farnia,was not involved in performing nol esters,hydrocarbons,rose oxide,and stearoptene.The the study verum was manufactured by Barij Essence Pharmaceutical At baseline,35 patients were assigned to the verum group Company in Kashan,Iran). and 33 were assigned to the placebo group.The two groups did not differ with respect to age(verum:mean age=32.45 years, Assessing SSRl-induced sexual dysfunction standard deviation =5.68 years;placebo:mean age To assess SSRI-I SD,after the thorough psychiatric inter- =34.02 years,standard deviation=6.45 years;t(66)=1.34,view,psychiatrists also explored the sexual dysfunctions of P=0.54),symptom severity,or sexual dysfunction each patient before he started the treatment with an SSRI, (Table 1).At follow-up,five patients dropped out of the at least 6 weeks before each patient entered the study,and verum group,and three dropped out of the placebo group. sexual dysfunction during the study.An SSRI-I SD was However,statistical computation was performed with the diagnosed in accordance with the DSM-5;3 if all other fac- intention-to-treat algorithm and not with the per-protocol tors were equal,sexual dysfunction emerged with the start algorithm. of SSRI intake. Table I Descriptive overview of the sexual dysfunction and depressive symptoms scores each group(verum versus placebo)for each assessment time (baseline,week 4,and week 8) Assessment times Baseline Week 4 Week 8 Group Verum Placebo Verum Placebo Verum Placebo N 35 33 35 33 35 33 M±SD M±SD M±SD M±SD M±SD M±SD Sexual drive 1.8±0.84 1.84±0.63 2.07±0.81 188±0.71 2.43±0.98 2.03±0.80 Erections 1.78±0.76 1.94±0.69 1.77±0.72 1.82±0.74 2.49±1.09 2.05±0.81 Ejaculations 1.89±0.79 1.92±0.68 2.01±0.78 188±0.72 2.71±1.10 2.2010.92 Problem assessment 1.79±0.99 1.84牡0.63 1.90±0.84 1.86±0.71 2.68±1.07 2.15±0.90 Overall satisfaction 1.71±0.89 1.88±0.64 2.14±0.91 1.90±0.68 2.68±1.11 2.33±1.05 Mean score 1.81±0.78 1.89±0.63 1.98±0.73 1.87±0.68 2.58±1.01 2.16±0.87 Beck Depression Inventory 18.93±5.48 18.45±5.43 16.03±475 18.33+5.30 Notes:The verum is from Rosa damascena.Week 8marks the end of the study. Abbreviations:N,number of subjects:M.mean:SD.standard deviation. Neuropsychiatric Disease and Treatment 2015:1I ubmit yo 629 DovepressNeuropsychiatric Disease and Treatment 2015:11 submit your manuscript | www.dovepress.com Dovepress Dovepress 629 Rosa damascena oil for male SSRI-induced sexual dysfunction previously; 2) he withdrew from the study; 3) he was taking any medication or drug that may affect sexual function; 4) he had any underlying medical or psychiatric disorder (except MDD) that may interfere with sexual function; or 5) he reported side effects (changes in physical and psychological well-being) related to adjuvant medication (intake of either the verum or the placebo). No side effects were reported at any time during the study. Of the 127 patients screened, 68 were randomly assigned either to the verum group or to the placebo group. Randomization occurred as follows: 35 blue (for verum) and 35 red (for placebo) chips were put in a ballot box and stirred; patients drew a chip and were then assigned to the corresponding group. Neither the patients nor the hospital staff responsible for the randomization knew the group to which any of the subjects had been assigned. Furthermore, none of the personnel involved in the study knew the group to which any of the patients had been assigned. The principal investigator, Vahid Farnia, was not involved in performing the study. At baseline, 35 patients were assigned to the verum group and 33 were assigned to the placebo group. The two groups did not differ with respect to age (verum: mean age =32.45 years, standard deviation =5.68 years; placebo: mean age =34.02 years, standard deviation =6.45 years; t(66)=1.34, P=0.54), symptom severity, or sexual dysfunction (Table 1). At follow-up, five patients dropped out of the verum group, and three dropped out of the placebo group. However, statistical computation was performed with the intention-to-treat algorithm and not with the per-protocol algorithm. Medication Patients took their standard SSRI-medications (duloxetine, escitalopram, venlafaxine, or sertraline). Dosages were individually adapted to patients and kept constant for 6 weeks prior to the start of the study in order to achieve treatment efficacy. Next, patients took either verum or placebo in the morning. The verum dosage was 2 mL/day and contained 17 mg Citronellol of essential oil of R. damascena (drops), whereas the placebo consisted of 2 mL/day of an oil–water solution with an identical scent. The verum and placebo flacons were identical in shape, weight, look, and, once opened, scent. (The verum was based on at least 5.8 mg citronellol in each mL of product; the active ingredients are citronellol, geraniol, nerol, linalool, and phenyl ethyl alcohol. Additional components include linalool, saturated fatty alcohols beta-phenyl-ethyl alcohol, farnesol, terpinene-1- ol-4, acetates of the indicated alcohols, free acids, aldehydes [fatty and aromatic], geranial, neral, ketones, phenols, phenol esters, hydrocarbons, rose oxide, and stearoptene. The verum was manufactured by Barij Essence Pharmaceutical Company in Kashan, Iran). Assessing SSRI-induced sexual dysfunction To assess SSRI-I SD, after the thorough psychiatric interview, psychiatrists also explored the sexual dysfunctions of each patient before he started the treatment with an SSRI, at least 6 weeks before each patient entered the study, and sexual dysfunction during the study. An SSRI-I SD was diagnosed in accordance with the DSM-5; 63 if all other factors were equal, sexual dysfunction emerged with the start of SSRI intake. Table 1 Descriptive overview of the sexual dysfunction and depressive symptoms scores each group (verum versus placebo) for each assessment time (baseline, week 4, and week 8) Assessment times Baseline Week 4 Week 8 Group Verum Placebo Verum Placebo Verum Placebo N 35 33 35 33 35 33 M ± SD M ± SD M ± SD M ± SD M ± SD M ± SD Sexual drive 1.8±0.84 1.84±0.63 2.07±0.81 1.88±0.71 2.43±0.98 2.03±0.80 Erections 1.78±0.76 1.94±0.69 1.77±0.72 1.82±0.74 2.49±1.09 2.05±0.81 Ejaculations 1.89±0.79 1.92±0.68 2.01±0.78 1.88±0.72 2.71±1.10 2.20±0.92 Problem assessment 1.79±0.99 1.84±0.63 1.90±0.84 1.86±0.71 2.68±1.07 2.15±0.90 Overall satisfaction 1.71±0.89 1.88±0.64 2.14±0.91 1.90±0.68 2.68±1.11 2.33±1.05 Mean score 1.81±0.78 1.89±0.63 1.98±0.73 1.87±0.68 2.58±1.01 2.16±0.87 Beck Depression Inventory 18.93±5.48 18.45±5.43 – – 16.03±4.75 18.33±5.30 Notes: The verum is from Rosa damascena. Week 8 marks the end of the study. Abbreviations: N, number of subjects; M, mean; SD, standard deviation