正在加载图片...
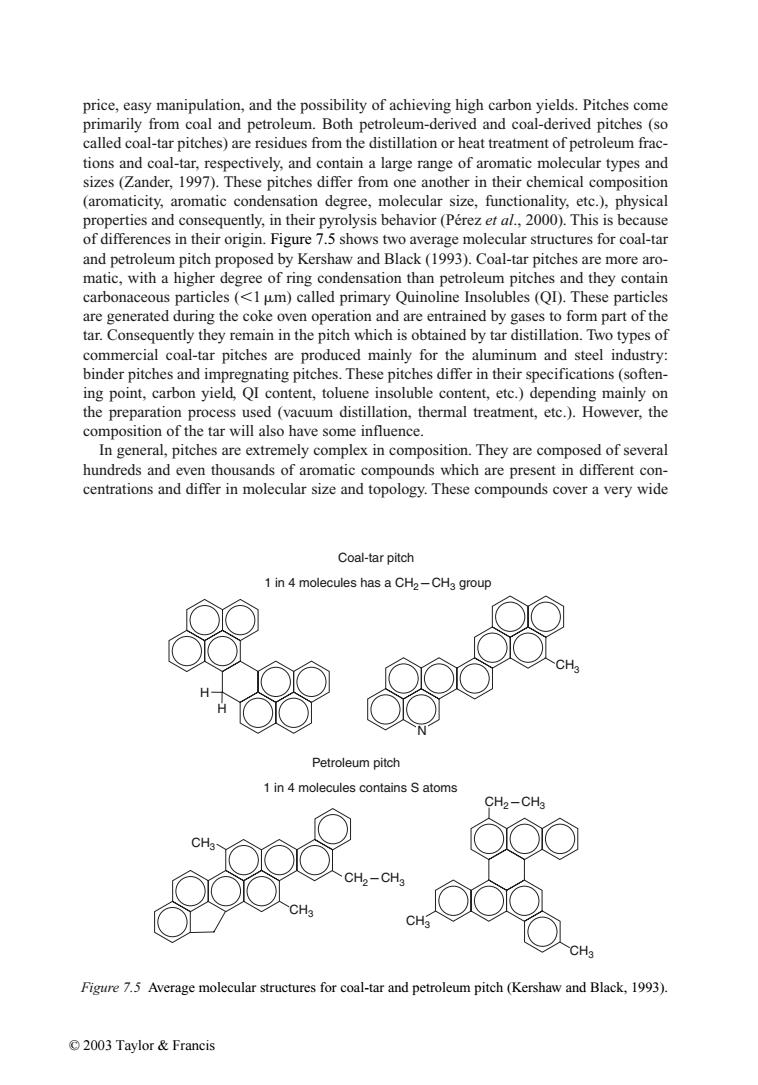
price,easy manipulation,and the possibility of achieving high carbon yields.Pitches come primarily from coal and petroleum.Both petroleum-derived and coal-derived pitches(so called coal-tar pitches)are residues from the distillation or heat treatment of petroleum frac- tions and coal-tar,respectively,and contain a large range of aromatic molecular types and sizes (Zander,1997).These pitches differ from one another in their chemical composition (aromaticity,aromatic condensation degree,molecular size,functionality,etc.),physical properties and consequently,in their pyrolysis behavior(Perez et al.,2000).This is because of differences in their origin.Figure 7.5 shows two average molecular structures for coal-tar and petroleum pitch proposed by Kershaw and Black(1993).Coal-tar pitches are more aro- matic,with a higher degree of ring condensation than petroleum pitches and they contain carbonaceous particles (<1 um)called primary Quinoline Insolubles (QD).These particles are generated during the coke oven operation and are entrained by gases to form part of the tar.Consequently they remain in the pitch which is obtained by tar distillation.Two types of commercial coal-tar pitches are produced mainly for the aluminum and steel industry: binder pitches and impregnating pitches.These pitches differ in their specifications(soften- ing point,carbon yield,QI content,toluene insoluble content,etc.)depending mainly on the preparation process used (vacuum distillation,thermal treatment,etc.).However,the composition of the tar will also have some influence. In general,pitches are extremely complex in composition.They are composed of several hundreds and even thousands of aromatic compounds which are present in different con- centrations and differ in molecular size and topology.These compounds cover a very wide Coal-tar pitch 1 in 4 molecules has a CH2-CH3 group Petroleum pitch 1 in 4 molecules contains S atoms CH2-CHa CH3 CH CH3 Figure 7.5 Average molecular structures for coal-tar and petroleum pitch (Kershaw and Black,1993). ©2003 Taylor&Francisprice, easy manipulation, and the possibility of achieving high carbon yields. Pitches come primarily from coal and petroleum. Both petroleum-derived and coal-derived pitches (so called coal-tar pitches) are residues from the distillation or heat treatment of petroleum fractions and coal-tar, respectively, and contain a large range of aromatic molecular types and sizes (Zander, 1997). These pitches differ from one another in their chemical composition (aromaticity, aromatic condensation degree, molecular size, functionality, etc.), physical properties and consequently, in their pyrolysis behavior (Pérez et al., 2000). This is because of differences in their origin. Figure 7.5 shows two average molecular structures for coal-tar and petroleum pitch proposed by Kershaw and Black (1993). Coal-tar pitches are more aromatic, with a higher degree of ring condensation than petroleum pitches and they contain carbonaceous particles (1m) called primary Quinoline Insolubles (QI). These particles are generated during the coke oven operation and are entrained by gases to form part of the tar. Consequently they remain in the pitch which is obtained by tar distillation. Two types of commercial coal-tar pitches are produced mainly for the aluminum and steel industry: binder pitches and impregnating pitches. These pitches differ in their specifications (softening point, carbon yield, QI content, toluene insoluble content, etc.) depending mainly on the preparation process used (vacuum distillation, thermal treatment, etc.). However, the composition of the tar will also have some influence. In general, pitches are extremely complex in composition. They are composed of several hundreds and even thousands of aromatic compounds which are present in different concentrations and differ in molecular size and topology. These compounds cover a very wide Figure 7.5 Average molecular structures for coal-tar and petroleum pitch (Kershaw and Black, 1993). H H N CH3 Coal-tar pitch CH3 CH3 CH3 CH3 Petroleum pitch 1 in 4 molecules contains S atoms 1 in 4 molecules has a CH2CH3 group CH2CH3 CH2CH3 © 2003 Taylor & Francis