正在加载图片...
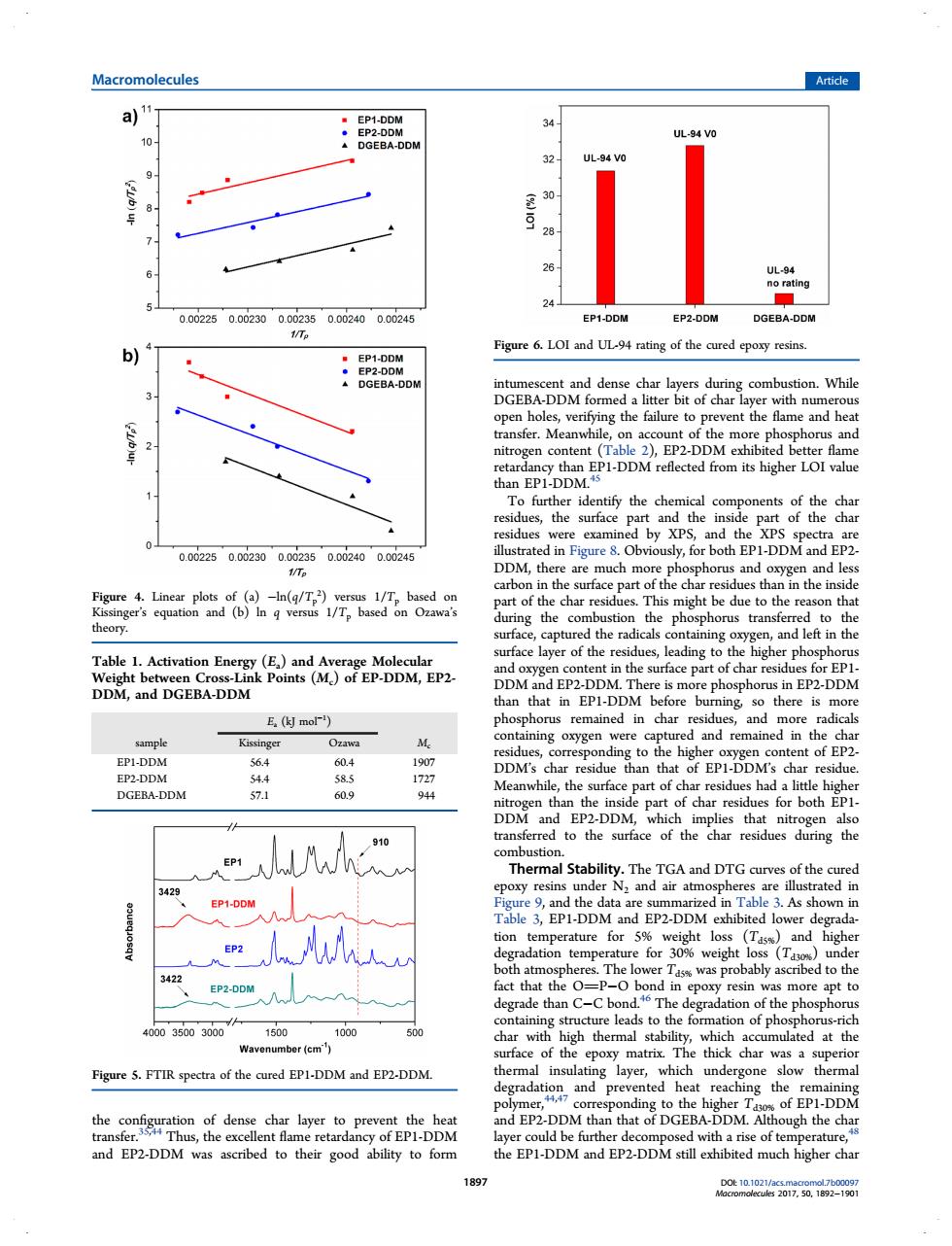
Macromolecules Article a)" 32 26 24 0.0225002300002350.002400.00245 EP1-DDM 1/Te b)4 Figure6.LOI ad UL rating of the cured epory resin of the more phe ohorus and thanp-DDM reflected from tts hgher LOI value a。o paboof the harredu thae DDM, 飘 This might tbe due to theres and left in th Table DDM,and DGEBA-DDM E.(kl mor) and rem EPI-DDM tent of EP2 604 19 esidue EP2-DDM Stability.The TGA and DTG of the cured poxy resins under N:and air atm Ta1-DDM and EPDDM ed lower and was pro ably a ed to th de than c C bor on of the 0003500300 cmulated at the ace epox Figure 5.FTIR spectra of the cured EP1-DDM and EP2-DDM. which degradation and prevented heat reachi ng the EPL-DM and EP2-DDM was ascribed to their good ability to form the EP1-DDMad EP2-DDMstill ehbited much higher charthe configuration of dense char layer to prevent the heat transfer.35,44 Thus, the excellent flame retardancy of EP1-DDM and EP2-DDM was ascribed to their good ability to form intumescent and dense char layers during combustion. While DGEBA-DDM formed a litter bit of char layer with numerous open holes, verifying the failure to prevent the flame and heat transfer. Meanwhile, on account of the more phosphorus and nitrogen content (Table 2), EP2-DDM exhibited better flame retardancy than EP1-DDM reflected from its higher LOI value than EP1-DDM.45 To further identify the chemical components of the char residues, the surface part and the inside part of the char residues were examined by XPS, and the XPS spectra are illustrated in Figure 8. Obviously, for both EP1-DDM and EP2- DDM, there are much more phosphorus and oxygen and less carbon in the surface part of the char residues than in the inside part of the char residues. This might be due to the reason that during the combustion the phosphorus transferred to the surface, captured the radicals containing oxygen, and left in the surface layer of the residues, leading to the higher phosphorus and oxygen content in the surface part of char residues for EP1- DDM and EP2-DDM. There is more phosphorus in EP2-DDM than that in EP1-DDM before burning, so there is more phosphorus remained in char residues, and more radicals containing oxygen were captured and remained in the char residues, corresponding to the higher oxygen content of EP2- DDM’s char residue than that of EP1-DDM’s char residue. Meanwhile, the surface part of char residues had a little higher nitrogen than the inside part of char residues for both EP1- DDM and EP2-DDM, which implies that nitrogen also transferred to the surface of the char residues during the combustion. Thermal Stability. The TGA and DTG curves of the cured epoxy resins under N2 and air atmospheres are illustrated in Figure 9, and the data are summarized in Table 3. As shown in Table 3, EP1-DDM and EP2-DDM exhibited lower degradation temperature for 5% weight loss (Td5%) and higher degradation temperature for 30% weight loss (Td30%) under both atmospheres. The lower Td5% was probably ascribed to the fact that the OP−O bond in epoxy resin was more apt to degrade than C−C bond.46 The degradation of the phosphorus containing structure leads to the formation of phosphorus-rich char with high thermal stability, which accumulated at the surface of the epoxy matrix. The thick char was a superior thermal insulating layer, which undergone slow thermal degradation and prevented heat reaching the remaining polymer,44,47 corresponding to the higher Td30% of EP1-DDM and EP2-DDM than that of DGEBA-DDM. Although the char layer could be further decomposed with a rise of temperature,48 the EP1-DDM and EP2-DDM still exhibited much higher char Figure 4. Linear plots of (a) −ln(q/Tp 2 ) versus 1/Tp based on Kissinger’s equation and (b) ln q versus 1/Tp based on Ozawa’s theory. Table 1. Activation Energy (Ea) and Average Molecular Weight between Cross-Link Points (Mc) of EP-DDM, EP2- DDM, and DGEBA-DDM Ea (kJ mol−1 ) sample Kissinger Ozawa Mc EP1-DDM 56.4 60.4 1907 EP2-DDM 54.4 58.5 1727 DGEBA-DDM 57.1 60.9 944 Figure 5. FTIR spectra of the cured EP1-DDM and EP2-DDM. Figure 6. LOI and UL-94 rating of the cured epoxy resins. Macromolecules Article DOI: 10.1021/acs.macromol.7b00097 Macromolecules 2017, 50, 1892−1901 1897