正在加载图片...
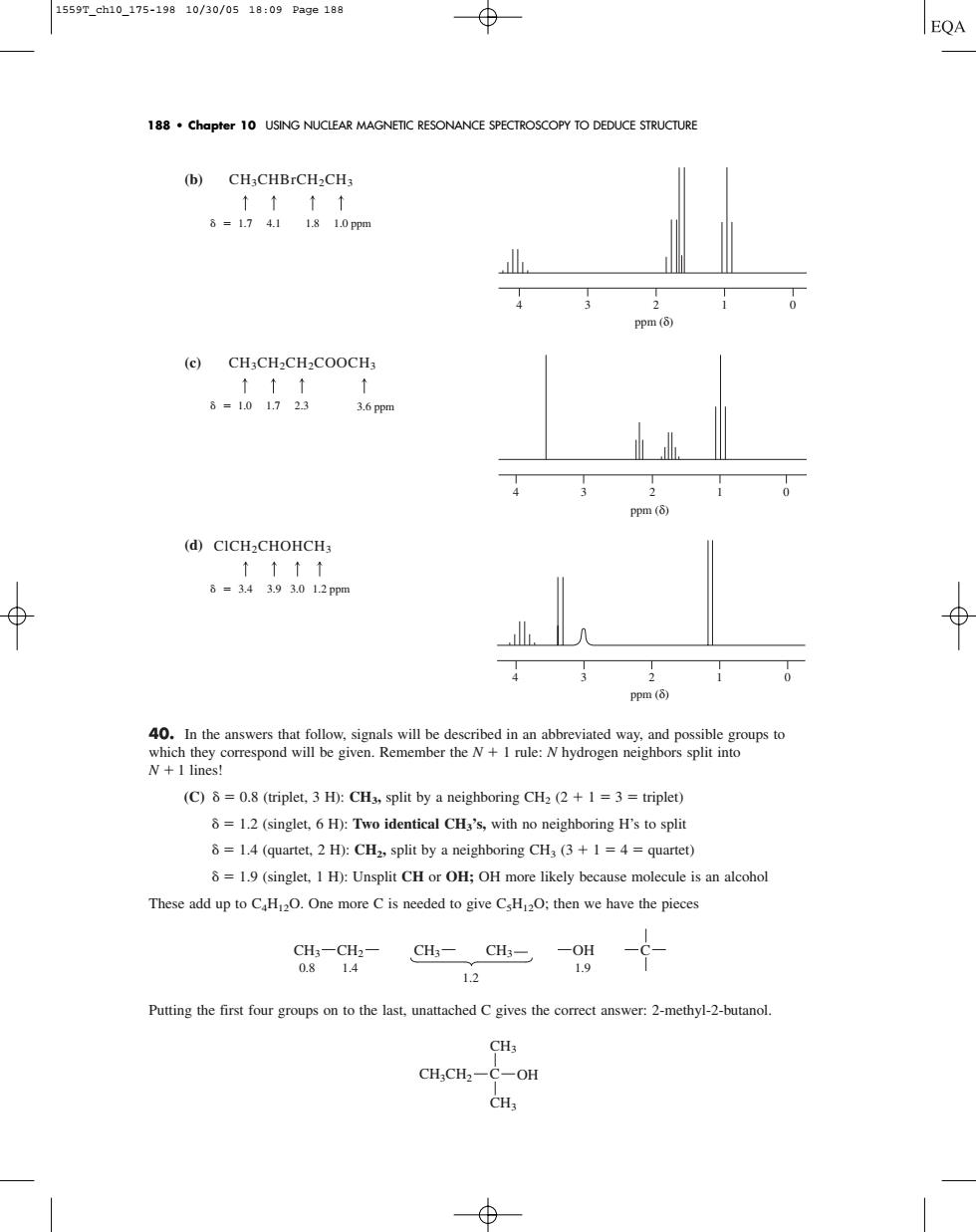
1559T_ch10_175-19810/30/0518:09Pa9e188 EQA 188.chapter 10 USING NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY TO DEDUCE STRUCTURE (b)CH3CHBrCH2CH3 ↑↑↑↑ 6-17411810pm 4 (c)CH:CH:CHCOOCH, t- 3.6pp (d④CICH2 CHOHCH ↑↑↑↑ 8=34393012pmm (C)=0.8 (triplet.3 H):CHa,split by a neighboring CH2 (2+1=3=triplet) =1.2(singlet.6 H):Two identical CH's,with no neighboring H's to split 8=1.4 (quartet.2 H):CH2,split by a neighboring CHa (3+1 =4=quartet) =1.9(singlet.I H):Unsplit CH or OH;OH more likely because molecule is an alcohol These add up to CH.One more Cis needed to give CsH2O.then we have the picces q-94 CHs-CHs-oy 12 Putting the first four groups on to the last.unattached Cgives the correct answer:2-methyl-2-butanol. CH3 CH,CH2-C-OH CH. (b) (c) (d) 40. In the answers that follow, signals will be described in an abbreviated way, and possible groups to which they correspond will be given. Remember the N 1 rule: N hydrogen neighbors split into N 1 lines! (C) 0.8 (triplet, 3 H): CH3, split by a neighboring CH2 (2 1 3 triplet) 1.2 (singlet, 6 H): Two identical CH3’s, with no neighboring H’s to split 1.4 (quartet, 2 H): CH2, split by a neighboring CH3 (3 1 4 quartet) 1.9 (singlet, 1 H): Unsplit CH or OH; OH more likely because molecule is an alcohol These add up to C4H12O. One more C is needed to give C5H12O; then we have the pieces Putting the first four groups on to the last, unattached C gives the correct answer: 2-methyl-2-butanol. CH3 CH3 CH3CH2 C OH CH3 0.8 1.4 1.9 1.2 CH2 CH3 CH3 OH C ClCH2CHOHCH3 3.4 3.9 3.0 1.2 ppm CH3CH2CH2COOCH3 1.0 1.7 2.3 3.6 ppm CH3CHBrCH2CH3 1.7 4.1 1.8 1.0 ppm 188 • Chapter 10 USING NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY TO DEDUCE STRUCTURE 4 3 2 1 0 ppm (δ) 4 3 2 1 0 ppm (δ) 4 3 2 1 0 ppm (δ) 1559T_ch10_175-198 10/30/05 18:09 Page 188�������