正在加载图片...
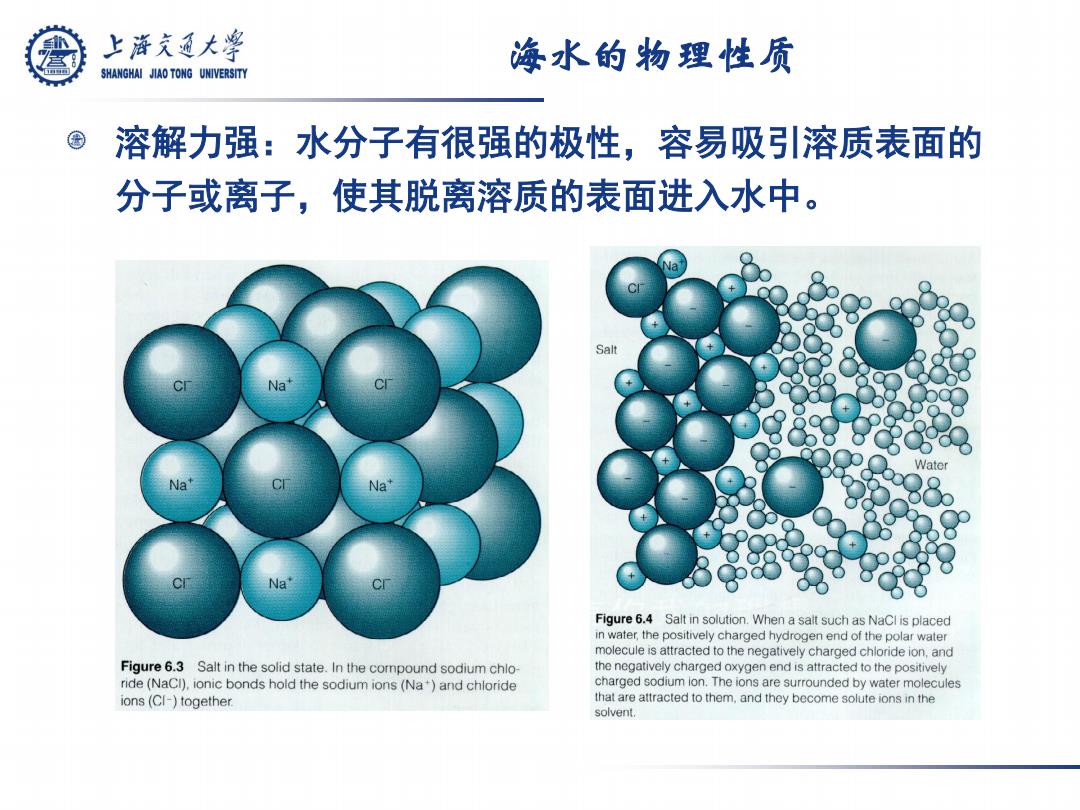
上游究通大学 海水的物理性质 SHANGHAI JIAO TONG UNIVERSITY 溶解力强:水分子有很强的极性,容易吸引溶质表面的 分子或离子,使其脱离溶质的表面进入水中。 Salt Na Na Figure 6.4 Salt in solution.When a salt such as NaCl is placed in water,the positively charged hydrogen end of the polar water molecule is attracted to the negatively charged chloride ion,and Figure 6.3 Salt in the solid state.In the compound sodium chlo- the negatively charged oxygen end is attracted to the positively ride(NaCI),ionic bonds hold the sodium ions (Na)and chloride charged sodium ion.The ions are surrounded by water molecules ions(CI-)together. that are attracted to them,and they become solute ions in the solvent.海水的物理性质 溶解力强:水分子有很强的极性,容易吸引溶质表面的 分子或离子,使其脱离溶质的表面进入水中