正在加载图片...
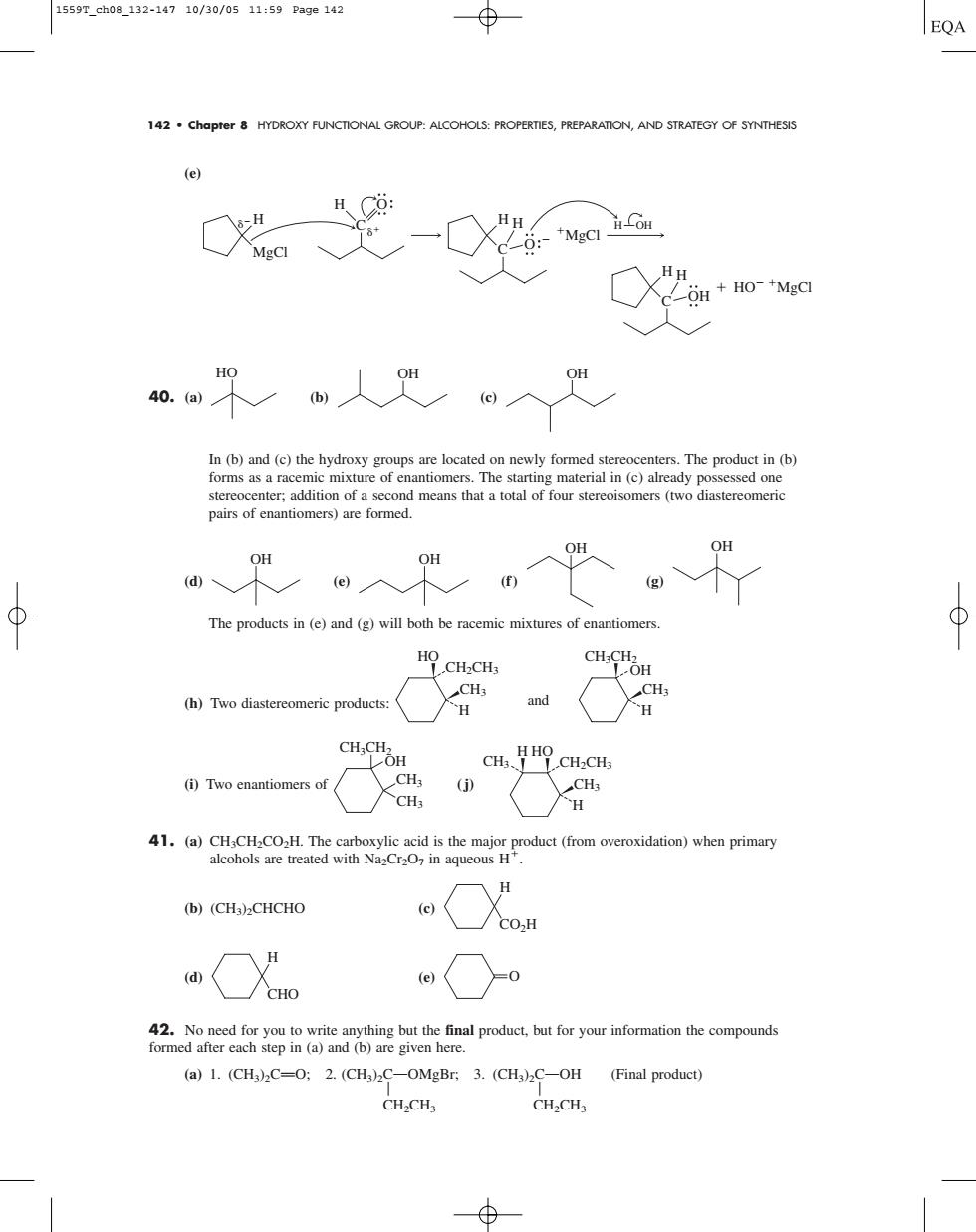
1559T_ch08_132-14710/30/0511:59Pa9e142 EQA 142.Chapter 8 HYDROXY FUNCTIONAL GROUP:ALCOHOLS:PROPERTIES,PREPARATION,AND STRATEGY OF SYNTHESIS (e) 0光-0 ○X+o In(b)nd (the hydryop oadomed The ro (b) stereocentr:addition of a second means that a total of four stereoisomers(two diastereomeric pairs of enantiomers)are formed. ⊕ The products in (e)and (g)will both beracemicmixtures of enantiomers CHCOH (h)Two diastereomeric products: CH.CHOH CH, CH.HOCH.CH (i)Two enantiomers of 4.a用C0 )sen pin (b)(CH3)2CHCHO OX oe a○ 2oaRa u to write a anything but the final product,but for your information the compounds (a)1.(CH)C-O:2.(CHa)2C-OMgBr.3.(CH3)2C-OH (Final product) CH.CHa 142 • Chapter 8 HYDROXY FUNCTIONAL GROUP: ALCOHOLS: PROPERTIES, PREPARATION, AND STRATEGY OF SYNTHESIS (e) 40. (a) (b) (c) In (b) and (c) the hydroxy groups are located on newly formed stereocenters. The product in (b) forms as a racemic mixture of enantiomers. The starting material in (c) already possessed one stereocenter; addition of a second means that a total of four stereoisomers (two diastereomeric pairs of enantiomers) are formed. (d) (e) (f ) (g) The products in (e) and (g) will both be racemic mixtures of enantiomers. (h) Two diastereomeric products: (i) Two enantiomers of (j) 41. (a) CH3CH2CO2H. The carboxylic acid is the major product (from overoxidation) when primary alcohols are treated with Na2Cr2O7 in aqueous H. (b) (CH3)2CHCHO (c) (d) (e) 42. No need for you to write anything but the final product, but for your information the compounds formed after each step in (a) and (b) are given here. (a) 1. (CH3)2CPO; 2. (CH3)2COOMgBr; 3. (CH3)2COOH (Final product) A A CH2CH3 CH2CH3 O CHO H CO2H H CH2CH3 CH3 CH3 HO H CH3CH2 H CH3 CH3 OH CH2CH3 CH3 HO H CH3CH2 CH3 OH H and OH OH OH OH HO OH OH C O C H H MgCl H H O MgCl C H H OH HO MgCl H OH 1559T_ch08_132-147 10/30/05 11:59 Page 142�������