正在加载图片...
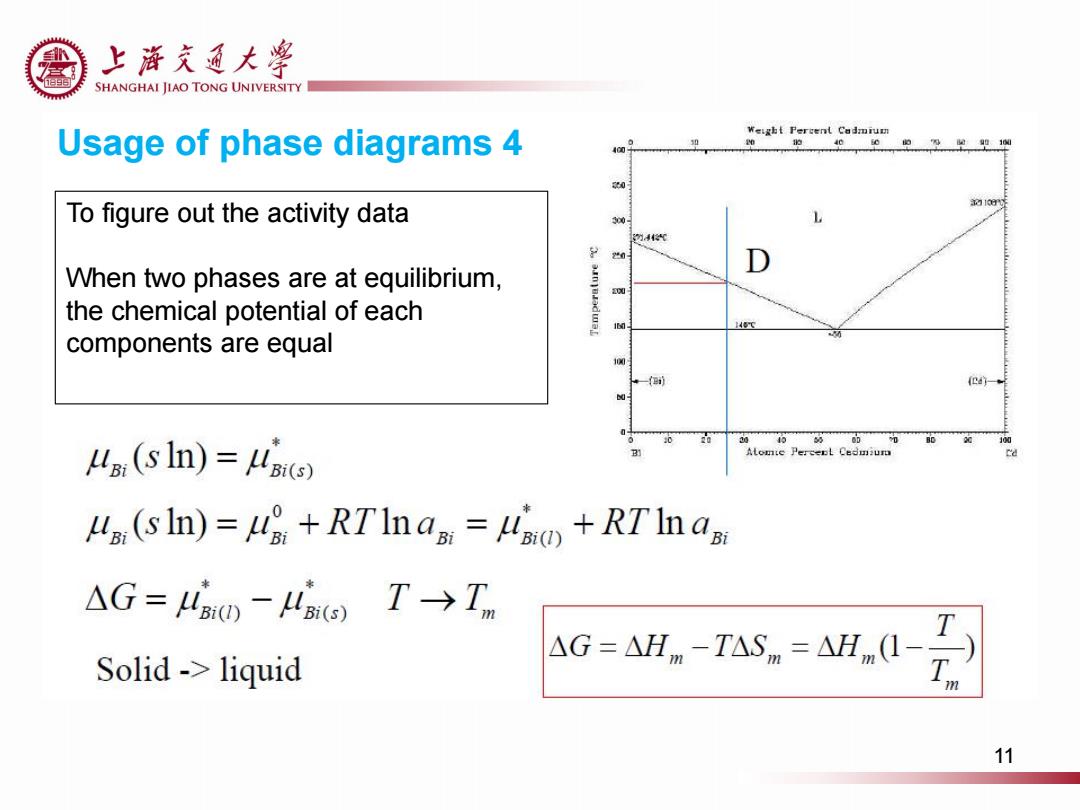
上游充通大粤 SHANGHAI JIAO TONG UNIVERSITY Usage of phase diagrams 4 4 To figure out the activity data 30 4代 20 When two phases are at equilibrium, the chemical potential of each components are equal (E4)- 100 a(s In)=) Lp:(s In)=uo+RTIn a=Li)+RT In a △G=4a0-4o T→Tm T Solid->liquid △G=△Hm-TASm=△Hm(I- m 11Usage of phase diagrams 4 To figure out the activity data When two phases are at equilibrium, the chemical potential of each components are equal 11