正在加载图片...
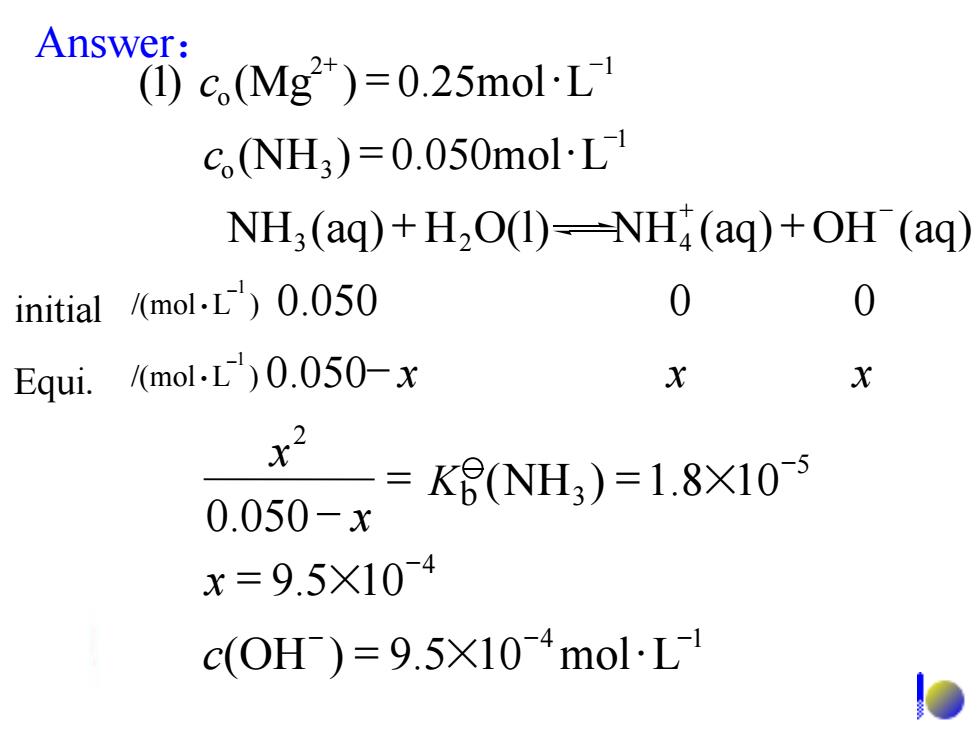
Answer: (1)c,(Mg2)=0.25molL1 cNH3)=0.050mol·L NH3 (aq)+H2O(1)=NH;(aq)+OH (aq) initial /(mol.L)0.050 0 Equi./(mol.L)0.050-x X X x2 =K6(NH3)=1.8×10 0.050-x x=9.5×10-4 c(0H)=9.5×104mol·L1c (NH ) 0.050mol L 1 o 3 = - (1) c (Mg ) 0.25mol L 2 1 o = + - /(mol L ) 0.050 x x x 1 - - Equi. /(mol L ) 0.050 0 0 -1 initial Answer: 4 1 (OH ) 9.5 10 mol L - - - c = × 4 9.5 10- x = × NH (aq) H O(l) NH (aq) OH (aq) 3 2 4 + + + - 5 3 2 (NH ) 1.8 10 0.050 - = = × - x x Kb