正在加载图片...
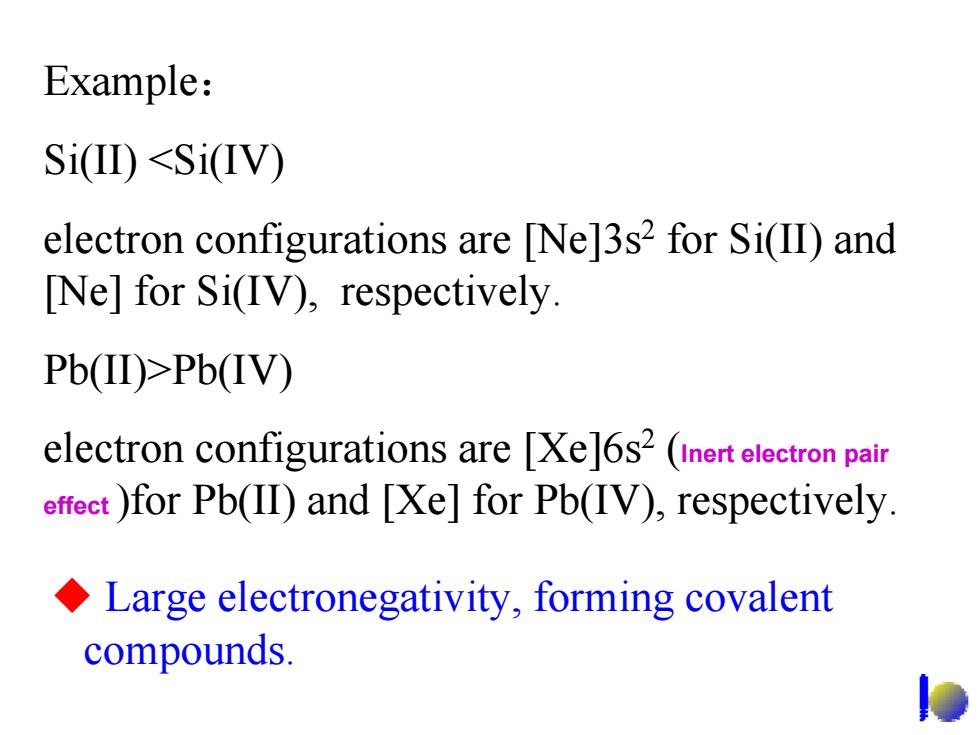
Example: Si(II)<SiIV) electron configurations are [Ne]3s2 for Si(II)and Ne]for Si(IV),respectively. Pb(II)>Pb(IV) electron configurations are [Xe]6s2 (inert electron pair effeet )for Pb(II)and [Xe]for Pb(IV),respectively. Large electronegativity,forming covalent compounds. Example: Si(II) <Si(IV) electron configurations are [Ne]3s2 for Si(II) and [Ne] for Si(IV), respectively. Pb(II)>Pb(IV) electron configurations are [Xe]6s2 (Inert electron pair effect )for Pb(II) and [Xe] for Pb(IV), respectively. Large electronegativity, forming covalent compounds