正在加载图片...
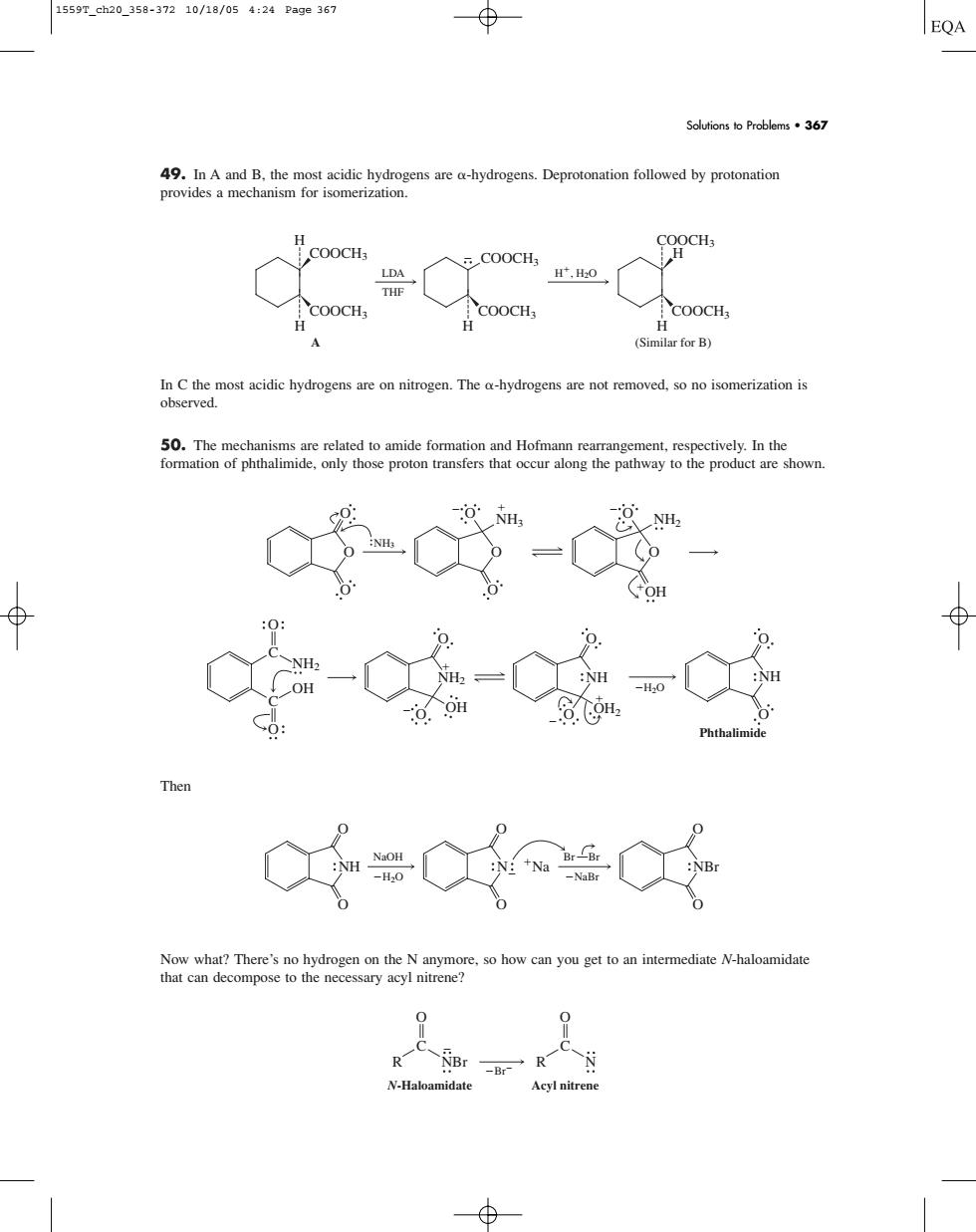
1559T_ch20_358-37210/18/054:24Pa9e36 EQA Solutions to Problems.367 9 d by protonatio 个co0cH for B) The a-hydrogens are not removed,so no isomerization is 一50.Te。ol those proto transrs thatth In th ⊕ C=00 what?The me 49. In A and B, the most acidic hydrogens are -hydrogens. Deprotonation followed by protonation provides a mechanism for isomerization. In C the most acidic hydrogens are on nitrogen. The -hydrogens are not removed, so no isomerization is observed. 50. The mechanisms are related to amide formation and Hofmann rearrangement, respectively. In the formation of phthalimide, only those proton transfers that occur along the pathway to the product are shown. Then Now what? There’s no hydrogen on the N anymore, so how can you get to an intermediate N-haloamidate that can decompose to the necessary acyl nitrene? Br O C R NBr N-Haloamidate O C R N Acyl nitrene O O NH O O N Na O O NBr H2O NaOH NaBr Br Br C C O O NH2 OH NH2 O OH NH OH2 O Phthalimide NH H2O O O O O O O O O NH3 NH3 O O NH2 O OH O LDA THF H, H2O COOCH3 COOCH3 H COOCH3 COOCH3 H H H A (Similar for B) H COOCH3 COOCH3 Solutions to Problems • 367 1559T_ch20_358-372 10/18/05 4:24 Page 367