正在加载图片...
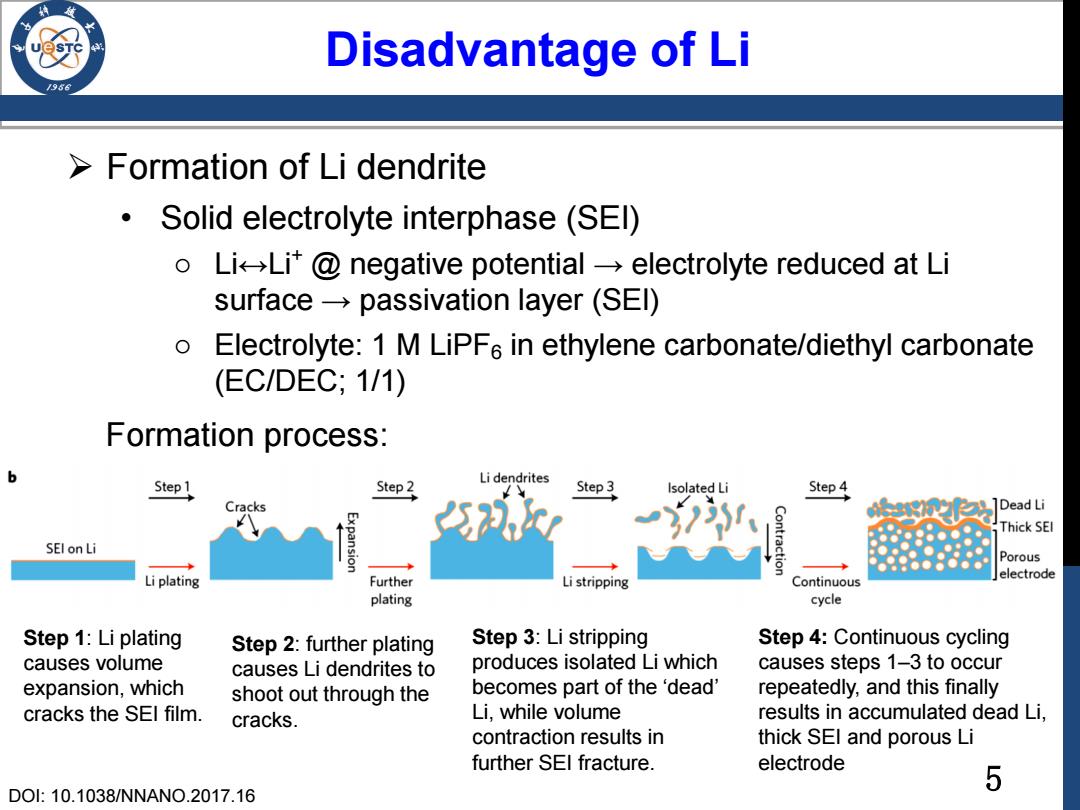
Disadvantage of Li Formation of Li dendrite Solid electrolyte interphase(SEl) o LiLit@negative potential-electrolyte reduced at Li surface-passivation layer (SEl) Electrolyte:1 M LiPF6 in ethylene carbonate/diethyl carbonate (EC/DEC;1/1) Formation process: b Li dendrites Step 1 Step 2 Step3 Isolated Li Step 4 Cracks ]Dead Li Thick SEI SEl on Li Porous Li plating Further Li stripping electrode Continuous plating cycle Step 1:Li plating Step 2:further plating Step 3:Li stripping Step 4:Continuous cycling causes volume causes Li dendrites to produces isolated Li which causes steps 1-3 to occur expansion,which shoot out through the becomes part of the 'dead' repeatedly,and this finally cracks the SEl film. cracks. Li,while volume results in accumulated dead Li, contraction results in thick SEl and porous Li further SEl fracture. electrode 5 DO:10.1038/NNANO.2017.165 Disadvantage of Li Formation of Li dendrite • Solid electrolyte interphase (SEI) o Li↔Li+ @ negative potential → electrolyte reduced at Li surface → passivation layer (SEI) o Electrolyte: 1 M LiPF6 in ethylene carbonate/diethyl carbonate (EC/DEC; 1/1) Step 1: Li plating causes volume expansion, which cracks the SEI film. DOI: 10.1038/NNANO.2017.16 Step 2: further plating causes Li dendrites to shoot out through the cracks. Step 3: Li stripping produces isolated Li which becomes part of the ‘dead’ Li, while volume contraction results in further SEI fracture. Step 4: Continuous cycling causes steps 1–3 to occur repeatedly, and this finally results in accumulated dead Li, thick SEI and porous Li electrode Formation process: