正在加载图片...
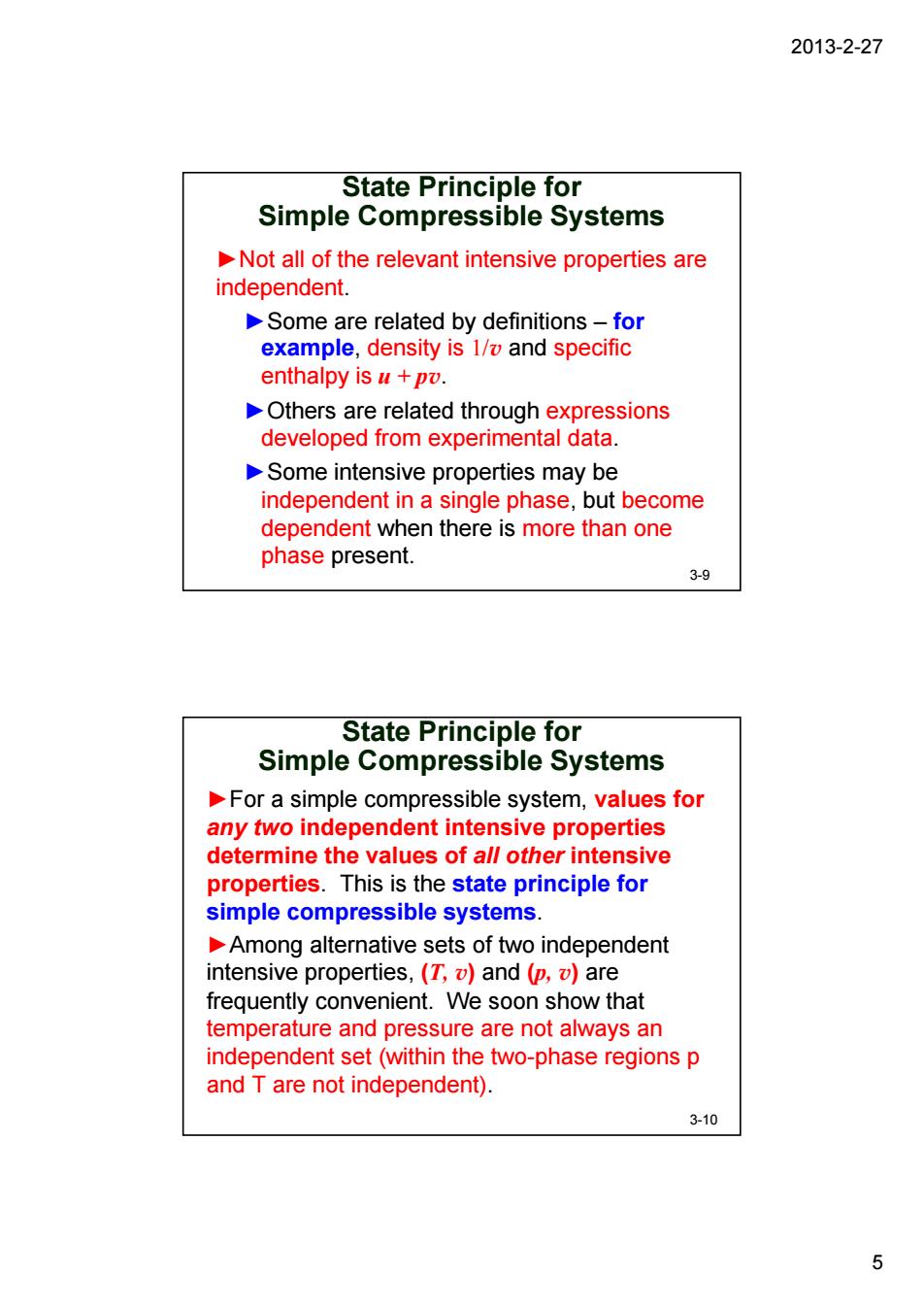
2013-2-27 State Principle for Simple Compressible Systems Not all of the relevant intensive properties are independent. Some are related by definitions-for example,density is 1/and specific enthalpy is u+pv. Others are related through expressions developed from experimental data. Some intensive properties may be independent in a single phase,but become dependent when there is more than one phase present. 3-9 State Principle for Simple Compressible Systems For a simple compressible system,values for any two independent intensive properties determine the values of al/other intensive properties.This is the state principle for simple compressible systems. Among alternative sets of two independent intensive properties,(T,and (p,are frequently convenient.We soon show that temperature and pressure are not always an independent set(within the two-phase regions p and T are not independent). 3-10 52013-2-27 5 State Principle for Simple Compressible Systems ►Not all of the relevant intensive properties are independent. ►Some are related by definitions – for example, density is 1/v and specific enthalpy is u + pv. ►Others are related through expressions developed from experimental data. ►Some intensive properties may be independent in a single phase, but become dependent when there is more than one phase present. 3-9 State Principle for Simple Compressible Systems ►For a simple compressible system, values for any two independent intensive properties determine the values of all other intensive properties. This is the state principle for simple compressible systems. ►Among alternative sets of two independent intensive properties, (T, v) and (p, v) are frequently convenient. We soon show that temperature and pressure are not always an independent set (within the two-phase regions p and T are not independent). 3-10