正在加载图片...
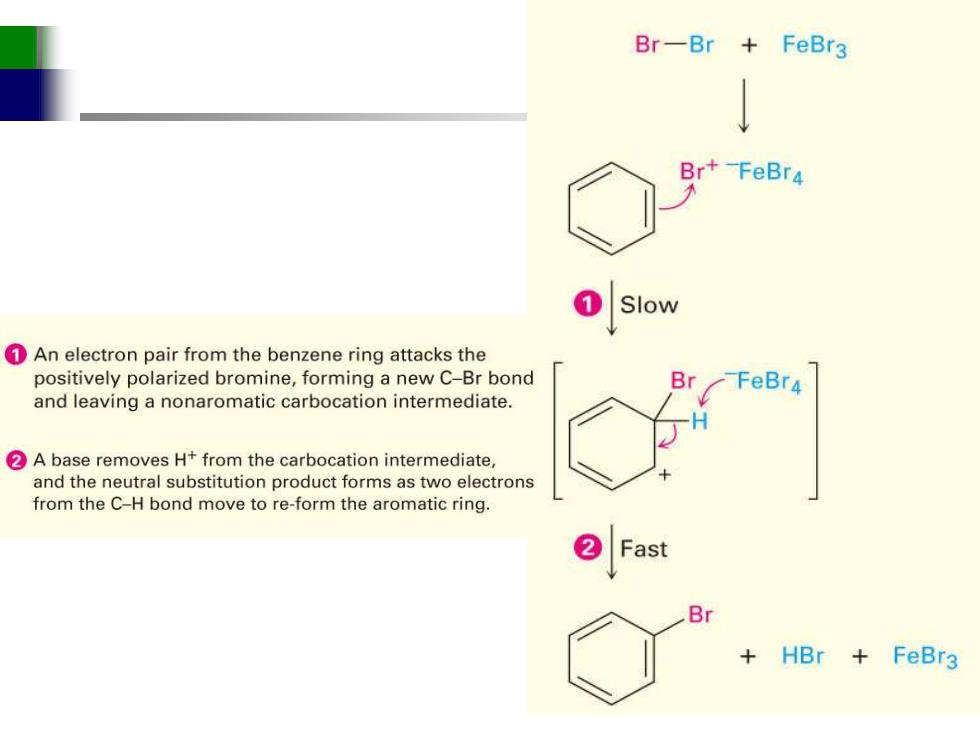
Br-Br+FeBr3 Br+-FeBr4 Slow 1An electron pair from the benzene ring attacks the positively polarized bromine,forming a new C-Br bond Br -FeBr4 and leaving a nonaromatic carbocation intermediate. 2A base removes H+from the carbocation intermediate, and the neutral substitution product forms as two electrons from the C-H bond move to re-form the aromatic ring. Fast Br +HBr+ FeBr3