正在加载图片...
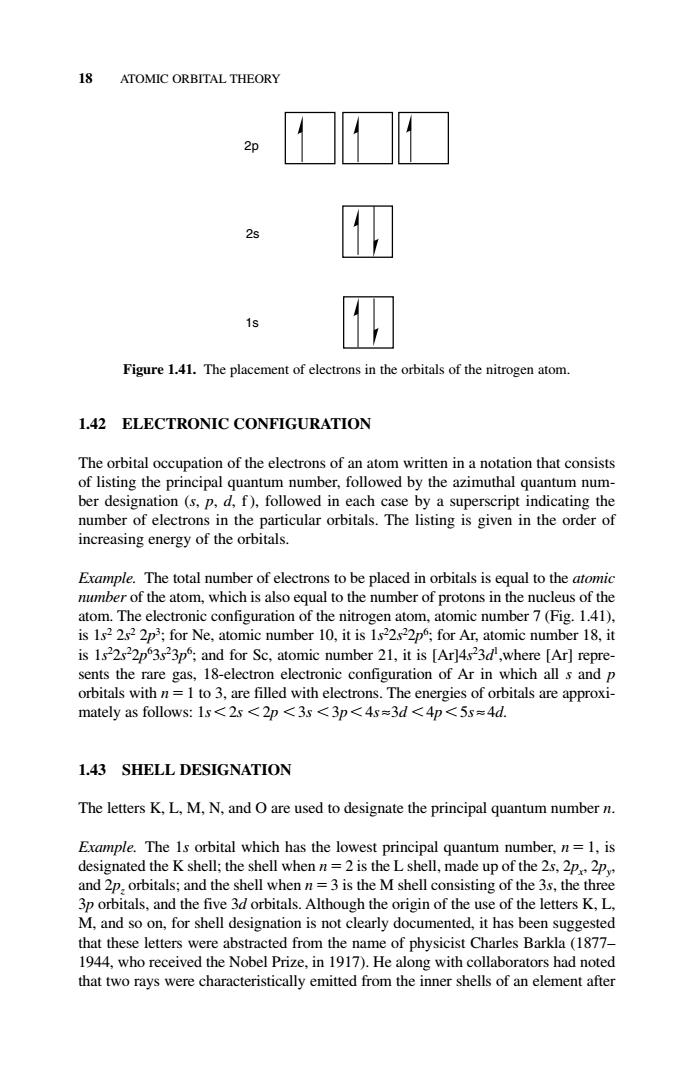
18 ATOMIC ORBITAL THEORY 29 Figure 1.41.The placement of electrons in the orbitals of the nitrogen atom 1.42 ELECTRONIC CONFIGURATION The orbital occupation of the electrons of an atom written in a notation that consists of listing the principal quantum number,followed by the azimuthal quantum num- ber designation (s.p,d,f).followed in each case by a superscript indicating the number of electrons in the particular orbitals.The listing is given in the order of increasing energy of the orbitals. Erample.The total numbe er of electrons to be placed in orbitals is equal to the atomi numbe of the atom,which is also equal to the number of protons in the nucleus of th atom.The electronic configuration of the nitrogen atom,atomic number 7(Fig.1.41). is 1s2 2s2 2p;for Ne,atomic number 10,it is 1s22s22p;for Ar,atomic number 18,it is 1s2222p53s23p;and for Sc.atomic number 21,it is [Ar)4523d,where [Ar]repre- sents the rare gas,18-electron electronic configuration of Ar in which all s and p 1.43 SHELL DESIGNATION The letters K,L,M,N.and O are used to designate the principal quantum number n. Eromple.The 1s orbital which has the lowest principal quantum number.n=1.is designated the K shell;the shell when n=2 is the L shell, ade up of the 2s.2 and 2p.orbitals;and the shell whe =3 is the M shell co of the 3s.the thr and the fi itals.Althoug h the for she deienation is not clearly domentd h en s e origin of he letters K. that these letters were abstracted from the name of physicist Charles Barkla(1877- 1944,who received the Nobel Prize,in 1917).He along with collaborators had noted that two rays were characteristically emitted from the inner shells of an element after 1.42 ELECTRONIC CONFIGURATION The orbital occupation of the electrons of an atom written in a notation that consists of listing the principal quantum number, followed by the azimuthal quantum number designation (s, p, d, f ), followed in each case by a superscript indicating the number of electrons in the particular orbitals. The listing is given in the order of increasing energy of the orbitals. Example. The total number of electrons to be placed in orbitals is equal to the atomic number of the atom, which is also equal to the number of protons in the nucleus of the atom. The electronic configuration of the nitrogen atom, atomic number 7 (Fig. 1.41), is 1s2 2s2 2p3 ; for Ne, atomic number 10, it is 1s2 2s2 2p6 ; for Ar, atomic number 18, it is 1s2 2s2 2p6 3s2 3p6 ; and for Sc, atomic number 21, it is [Ar]4s2 3d1 ,where [Ar] represents the rare gas, 18-electron electronic configuration of Ar in which all s and p orbitals with n 1 to 3, are filled with electrons. The energies of orbitals are approximately as follows: 1s 2s 2p 3s 3p 4s ≈3d 4p 5s ≈ 4d. 1.43 SHELL DESIGNATION The letters K, L, M, N, and O are used to designate the principal quantum number n. Example. The 1s orbital which has the lowest principal quantum number, n 1, is designated the K shell; the shell when n 2 is the L shell, made up of the 2s, 2px, 2py, and 2pz orbitals; and the shell when n 3 is the M shell consisting of the 3s, the three 3p orbitals, and the five 3d orbitals. Although the origin of the use of the letters K, L, M, and so on, for shell designation is not clearly documented, it has been suggested that these letters were abstracted from the name of physicist Charles Barkla (1877– 1944, who received the Nobel Prize, in 1917). He along with collaborators had noted that two rays were characteristically emitted from the inner shells of an element after 18 ATOMIC ORBITAL THEORY 1s 2s 2p Figure 1.41. The placement of electrons in the orbitals of the nitrogen atom. c01.qxd 5/17/2005 5:12 PM Page 18