正在加载图片...
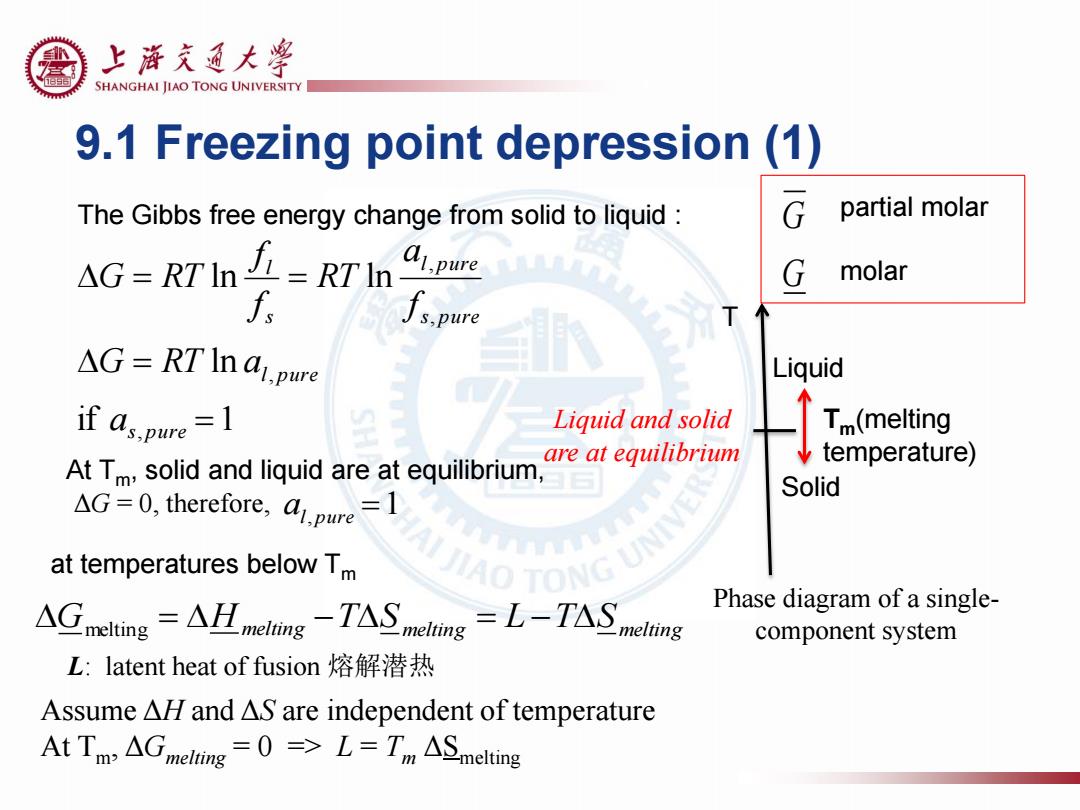
上游充通大 SHANGHAI JIAO TONG UNIVERSITY 9.1 Freezing point depression(1) The Gibbs free energy change from solid to liquid G partial molar AG=RTn∠=RTIn al,pure G molar f s,pure △G=RT In a,pre Liquid 个 if as.pure=1 Liquid and solid Tm(melting At Tm,solid and liquid are at equilibrium, are at equilibrium temperature) Solid AG=0,therefore,apure=1 at temperatures below Tm IAO TONG UNI AGmelting=AH nelong-TASmcling =L-TAS Phase diagram of a single- melting component system L:latent heat of fusion熔解潜热 Assume AH and AS are independent of temperature AtTm,△melring=0=>L=Tm△Smelting9.1 Freezing point depression (1) if 1 ln ln ln , , , , s pure l pure s pure l pure s l a G RT a f a RT f f G RT Tm(melting temperature) T Liquid Solid Phase diagram of a singlecomponent system Liquid and solid are at equilibrium The Gibbs free energy change from solid to liquid : At Tm, solid and liquid are at equilibrium, ΔG = 0, therefore, Gmelting Hmelting TS melting L TS melting al, pure 1 L: latent heat of fusion 熔解潜热 at temperatures below Tm Assume ΔH and ΔS are independent of temperature At Tm, ΔGmelting = 0 => L = Tm ΔSmelting G G partial molar molar