正在加载图片...
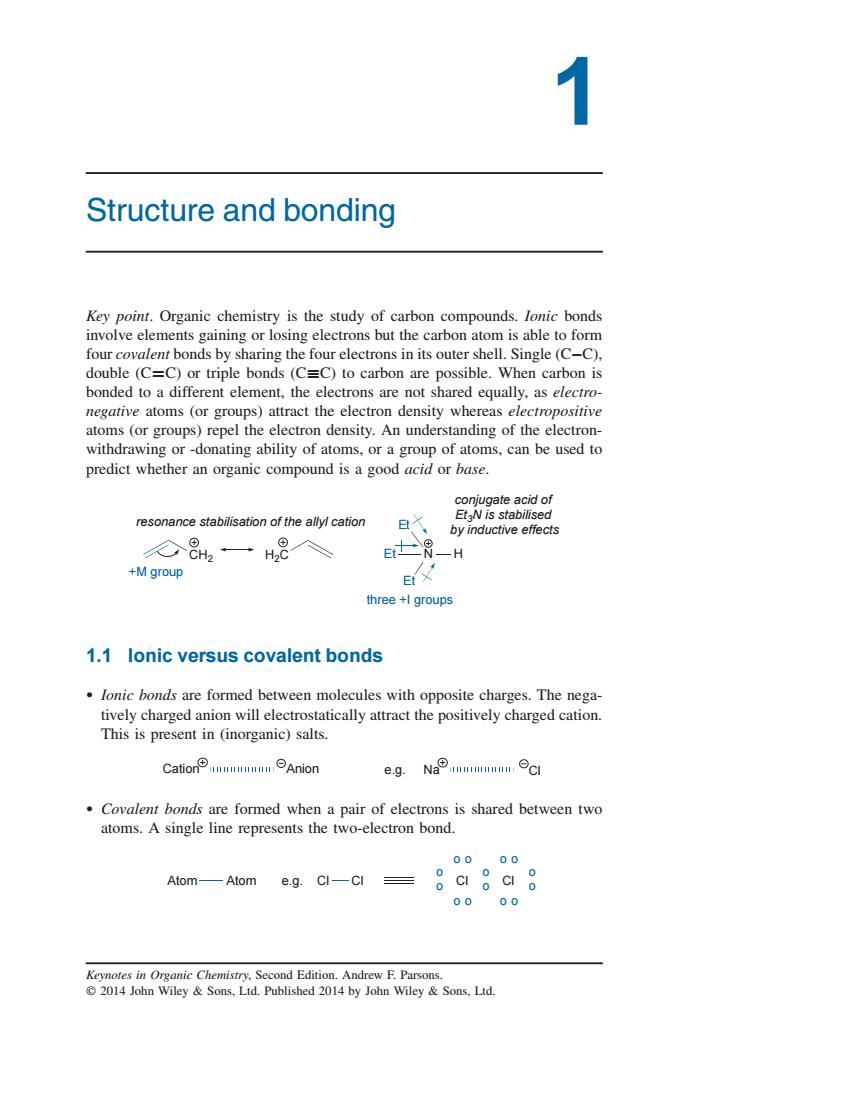
Structure and bonding Key point.Organic chemistry is the study of carbon compounds.lonic bonds involve elements gaining or losing electrons but the carbon atom is able to form four covalent bonds by sharing the four electrons in its outer shell.Single (C-C). double (C=C)or triple bonds(C=C)to carbon are possible.When carbon is bonded to a different element,the electrons are not shared equally.as electro- negative atoms (or groups)attract the electron density whereas electropositive atoms (or groups)repel the electron density.An understanding of the electron- withdrawing or-donating ability of atoms,or a group of atoms,can be used to predict whether an organic compound is a good base. resonance stabilisation of the allyl cation by inductive effed 8一H8入 e -H +M group three+lgroups 1.1 lonic versus covalent bonds lonic bonds are formed between molecules with opposite charges.The nega- eheernaianateondytheadeaia e.g.Na Covalent bonds are formed when a pair of electrons is shared between two atoms.A single line represents the two-electron bond. 0 Atom sg.GI-CI=。gag Aom— 0000 1 Structure and bonding Key point. Organic chemistry is the study of carbon compounds. Ionic bonds involve elements gaining or losing electrons but the carbon atom is able to form four covalent bonds by sharing the four electrons in its outer shell. Single (CC), double (CC) or triple bonds (CC) to carbon are possible. When carbon is bonded to a different element, the electrons are not shared equally, as electronegative atoms (or groups) attract the electron density whereas electropositive atoms (or groups) repel the electron density. An understanding of the electronwithdrawing or -donating ability of atoms, or a group of atoms, can be used to predict whether an organic compound is a good acid or base. CH2 H2C resonance stabilisation of the allyl cation +M group Et Et N Et H three +I groups conjugate acid of Et3N is stabilised by inductive effects 1.1 Ionic versus covalent bonds Ionic bonds are formed between molecules with opposite charges. The negatively charged anion will electrostatically attract the positively charged cation. This is present in (inorganic) salts. Cation Anion e.g. Na Cl Covalent bonds are formed when a pair of electrons is shared between two atoms. A single line represents the two-electron bond. Atom Atom Cl Cl e.g. o o o o o o Cl o o o o o o Cl o o Keynotes in Organic Chemistry, Second Edition. Andrew F. Parsons. 2014 John Wiley & Sons, Ltd. Published 2014 by John Wiley & Sons, Ltd.��