正在加载图片...
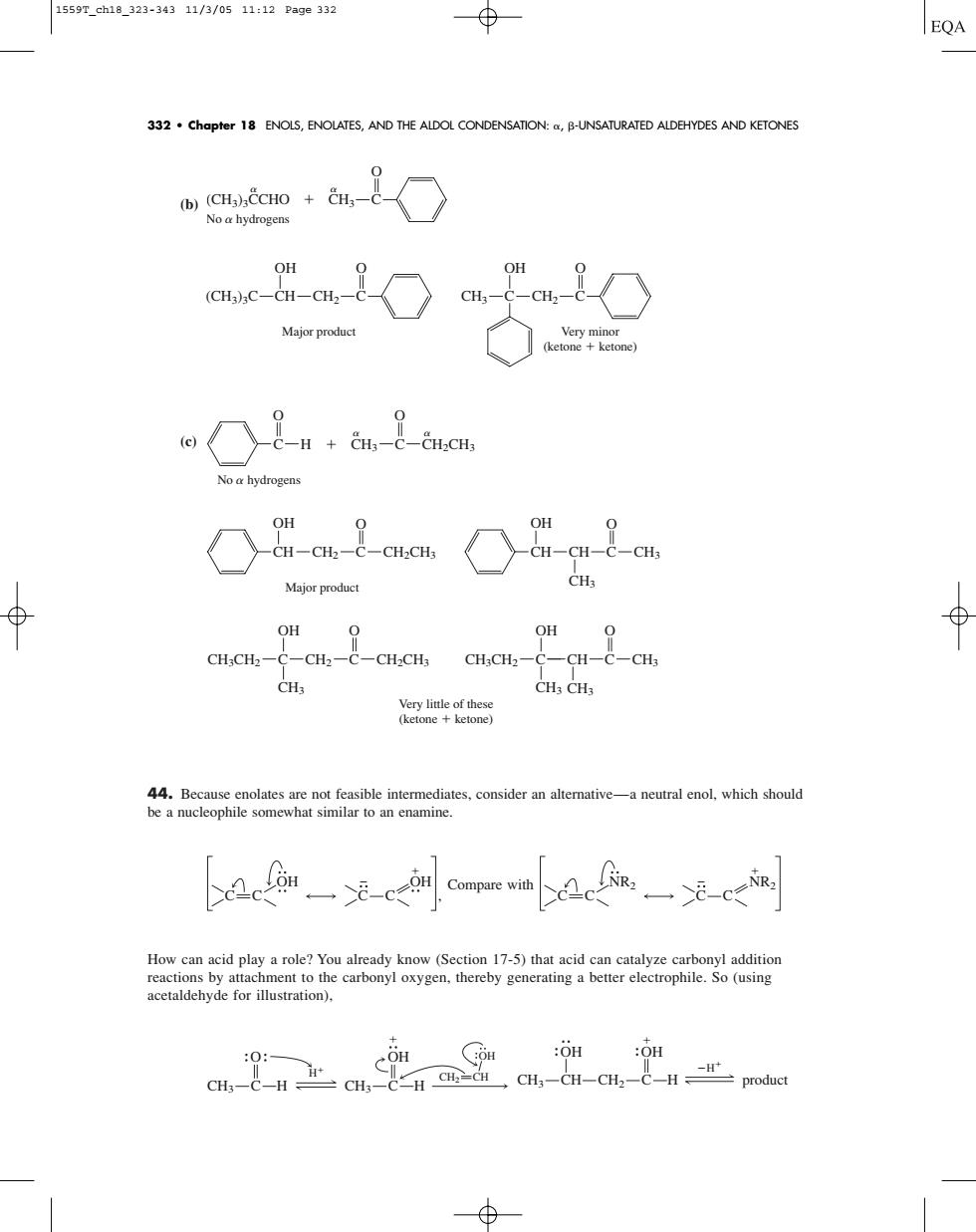
1559T_ch18_323-34311/3/0511:12Page332 EQA 332.Chapter 18 ENOLS,ENOLATES,AND THE ALDOL CONDENSATION:B-UNSATURATED ALDEHYDES AND KETONES wSae+a8○ No a hydrogens in-inC o a hydroge Major product OH O OH O :-CH2-C-CHCHa CH:CH2-C-CH-C-CH acetaldehyde for illustration). :OH :OH CH CH-CH-CH-C product (b) (c) 44. Because enolates are not feasible intermediates, consider an alternative—a neutral enol, which should be a nucleophile somewhat similar to an enamine. Compare with How can acid play a role? You already know (Section 17-5) that acid can catalyze carbonyl addition reactions by attachment to the carbonyl oxygen, thereby generating a better electrophile. So (using acetaldehyde for illustration), OH CH3 CH CH2 OH C H H product O OH CH3 C H CH2 CH OH CH3 C H H NR2 NR2 C C C C OH C C OH C C , O CH2 C CH2CH3 O CH3 C CH2CH3 CH3 CH OH Very little of these (ketone ketone) Major product O C H No hydrogens O C CH3 CH CH OH O CH2 C CH2CH3 CH3 C OH CH3CH2 CH3 C OH CH3CH2 CH3 O CH C CH3 C O CH3 C CH2 OH C O (CH3)3C CH CH2 OH Very minor (ketone ketone) Major product C O (CH3)3CCHO CH3 No hydrogens 332 • Chapter 18 ENOLS, ENOLATES, AND THE ALDOL CONDENSATION: , -UNSATURATED ALDEHYDES AND KETONES 1559T_ch18_323-343 11/3/05 11:12 Page 332�