正在加载图片...
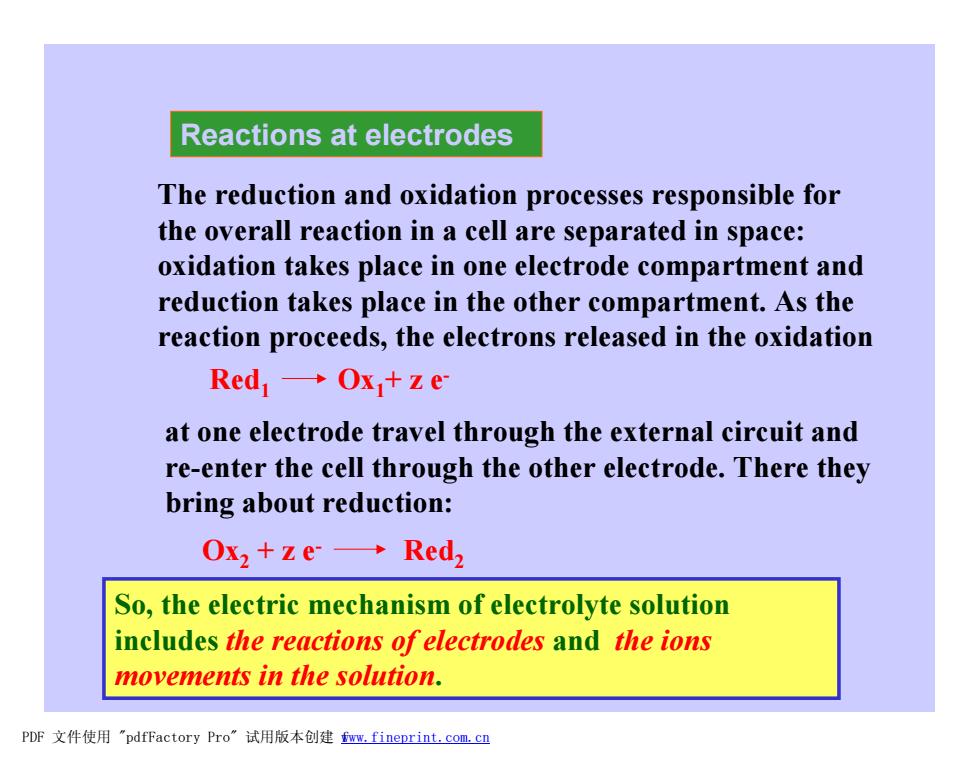
Reactions at electrodes The reduction and oxidation processes responsible for the overall reaction in a cell are separated in space: oxidation takes place in one electrode compartment and reduction takes place in the other compartment.As the reaction proceeds,the electrons released in the oxidation Red1→Ox+ze at one electrode travel through the external circuit and re-enter the cell through the other electrode.There they bring about reduction: Ox2+ze→Red So,the electric mechanism of electrolyte solution includes the reactions of electrodes and the ions movements in the solution. PDF文件使用"pdfFactory Pro”试用版本创建fww.fineprint.com.cn Reactions at electrodes The reduction and oxidation processes responsible for the overall reaction in a cell are separated in space: oxidation takes place in one electrode compartment and reduction takes place in the other compartment. As the reaction proceeds, the electrons released in the oxidation Red1 Ox1+ z eat one electrode travel through the external circuit and re-enter the cell through the other electrode. There they bring about reduction: Ox2 + z e- Red2 So, the electric mechanism of electrolyte solution includes the reactions of electrodes and the ions movements in the solution. PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn