正在加载图片...
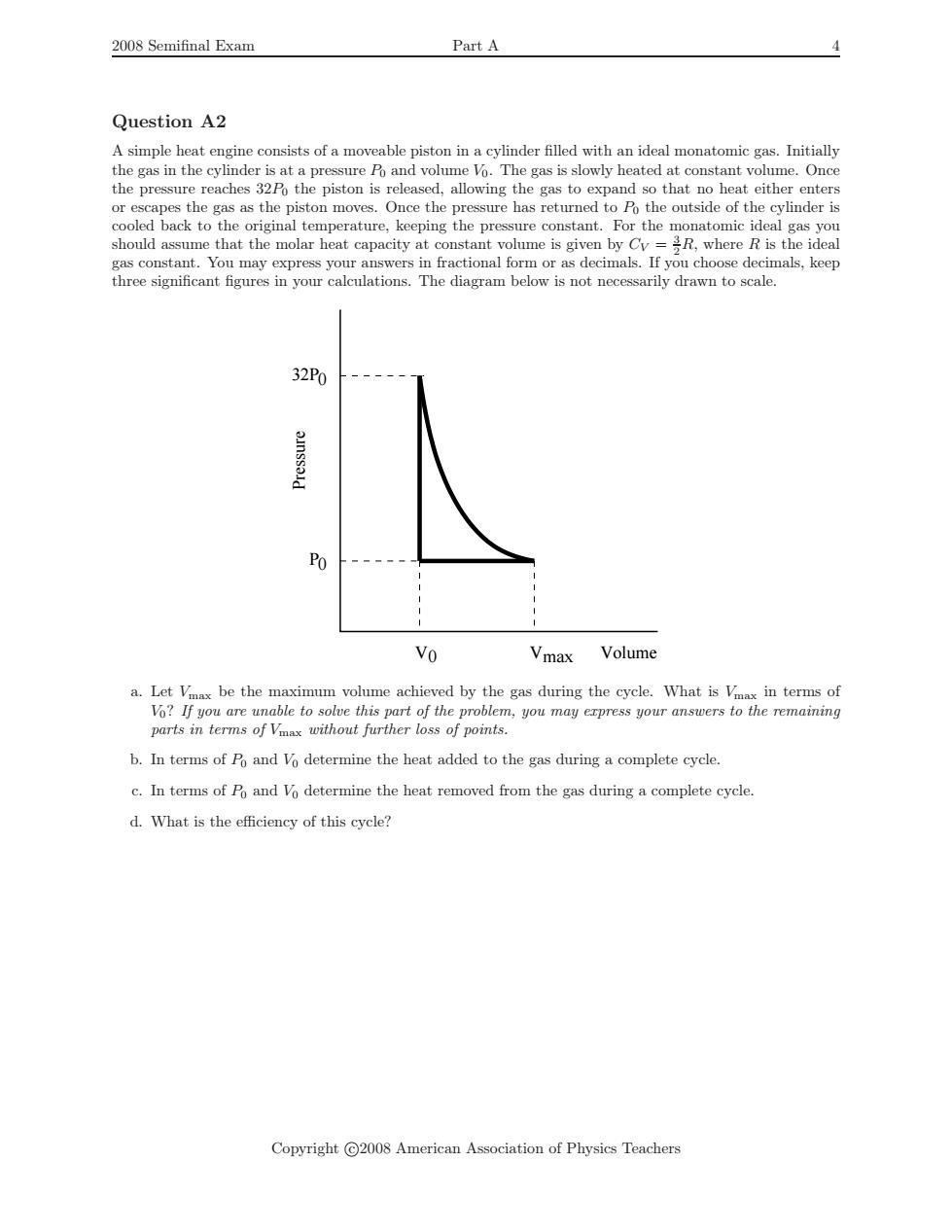
2008 Semifinal Exam Part A Question A2 A simple heat engine consists of a moveable piston in a cylinder filled with an ideal monatomic gas.Initially the gas in the cylinder is at a pressure Po and volume Vo.The gas is slowly heated at constant volume.Once the pressure reaches 32Po the piston is released,allowing the gas to expand so that no heat either enters or escapes the gas as the piston moves.Once the pressure has returned to Po the outside of the cylinder is cooled back to the original temperature,keeping the pressure constant.For the monatomic ideal gas you should assume that the molar heat capacity at constant volume is given by Cy=R,where R is the ideal gas constant.You may express your answers in fractional form or as decimals.If you choose decimals,keep three significant figures in your calculations.The diagram below is not necessarily drawn to scale. 32P0 Po Vo Vmax Volume a.Let Vax be the maximum volume achieved by the gas during the cycle.What is Vax in terms of Vo?If you are unable to solve this part of the problem,you may express your answers to the remaining parts in terms of Vmax without further loss of points. b.In terms of Po and Vo determine the heat added to the gas during a complete cycle. c.In terms of Po and Vo determine the heat removed from the gas during a complete cycle. d.What is the efficiency of this cycle? Copyright C2008 American Association of Physics Teachers2008 Semifinal Exam Part A 4 Question A2 A simple heat engine consists of a moveable piston in a cylinder filled with an ideal monatomic gas. Initially the gas in the cylinder is at a pressure P0 and volume V0. The gas is slowly heated at constant volume. Once the pressure reaches 32P0 the piston is released, allowing the gas to expand so that no heat either enters or escapes the gas as the piston moves. Once the pressure has returned to P0 the outside of the cylinder is cooled back to the original temperature, keeping the pressure constant. For the monatomic ideal gas you should assume that the molar heat capacity at constant volume is given by CV = 3 2R, where R is the ideal gas constant. You may express your answers in fractional form or as decimals. If you choose decimals, keep three significant figures in your calculations. The diagram below is not necessarily drawn to scale. Pressure P 0 0 V0 32P Vmax Volume a. Let Vmax be the maximum volume achieved by the gas during the cycle. What is Vmax in terms of V0? If you are unable to solve this part of the problem, you may express your answers to the remaining parts in terms of Vmax without further loss of points. b. In terms of P0 and V0 determine the heat added to the gas during a complete cycle. c. In terms of P0 and V0 determine the heat removed from the gas during a complete cycle. d. What is the efficiency of this cycle? Copyright c 2008 American Association of Physics Teachers