正在加载图片...
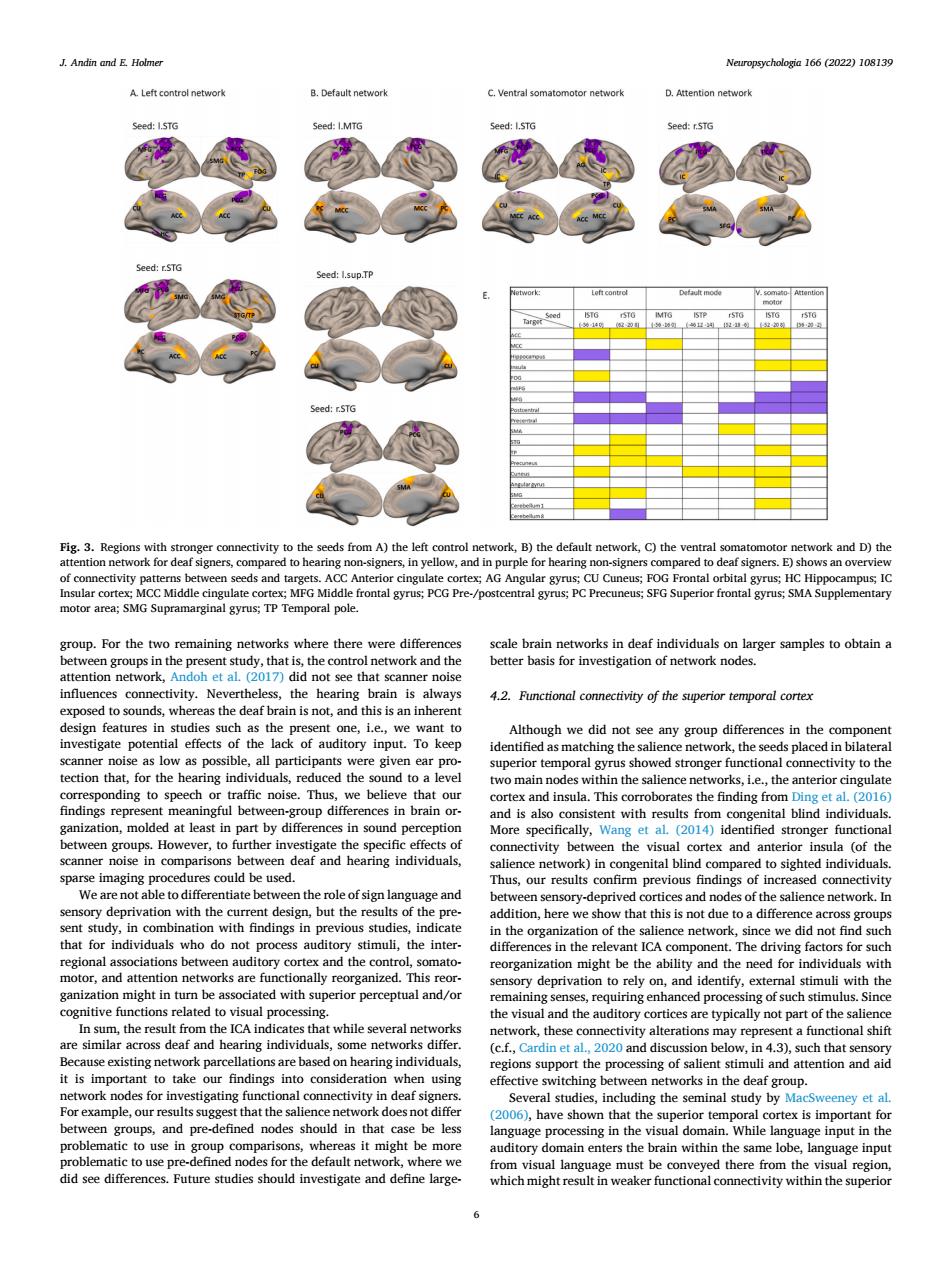
J.Andin and E Holmer Neuropsycholegin 166 (2022)108139 group.For the twe emaining networks where there were diff ot see that scar as the deaf brain is not.and this is an nen uperiortempoalgyrushovedtromgerfnctionalconmetiytoth or funet &e compatort nity be spaWearenotabtetodiierentiaiebetwent由eroleofsignlangy group hat sumun,the inte ant ICA component The driving factors for suc tor,and at a y on.and identify.ex mal stimuli with the pen nal proces deaf individua orks differ in 4 consideralion8t the and attention and aic ork nodes 1o ey et a engroups,and pre-defined nodes should in that be les ar in in the visul domain.While langt e must be c unctNeuropsychologia 166 (2022) 108139 6 group. For the two remaining networks where there were differences between groups in the present study, that is, the control network and the attention network, Andoh et al. (2017) did not see that scanner noise influences connectivity. Nevertheless, the hearing brain is always exposed to sounds, whereas the deaf brain is not, and this is an inherent design features in studies such as the present one, i.e., we want to investigate potential effects of the lack of auditory input. To keep scanner noise as low as possible, all participants were given ear protection that, for the hearing individuals, reduced the sound to a level corresponding to speech or traffic noise. Thus, we believe that our findings represent meaningful between-group differences in brain organization, molded at least in part by differences in sound perception between groups. However, to further investigate the specific effects of scanner noise in comparisons between deaf and hearing individuals, sparse imaging procedures could be used. We are not able to differentiate between the role of sign language and sensory deprivation with the current design, but the results of the present study, in combination with findings in previous studies, indicate that for individuals who do not process auditory stimuli, the interregional associations between auditory cortex and the control, somatomotor, and attention networks are functionally reorganized. This reorganization might in turn be associated with superior perceptual and/or cognitive functions related to visual processing. In sum, the result from the ICA indicates that while several networks are similar across deaf and hearing individuals, some networks differ. Because existing network parcellations are based on hearing individuals, it is important to take our findings into consideration when using network nodes for investigating functional connectivity in deaf signers. For example, our results suggest that the salience network does not differ between groups, and pre-defined nodes should in that case be less problematic to use in group comparisons, whereas it might be more problematic to use pre-defined nodes for the default network, where we did see differences. Future studies should investigate and define largescale brain networks in deaf individuals on larger samples to obtain a better basis for investigation of network nodes. 4.2. Functional connectivity of the superior temporal cortex Although we did not see any group differences in the component identified as matching the salience network, the seeds placed in bilateral superior temporal gyrus showed stronger functional connectivity to the two main nodes within the salience networks, i.e., the anterior cingulate cortex and insula. This corroborates the finding from Ding et al. (2016) and is also consistent with results from congenital blind individuals. More specifically, Wang et al. (2014) identified stronger functional connectivity between the visual cortex and anterior insula (of the salience network) in congenital blind compared to sighted individuals. Thus, our results confirm previous findings of increased connectivity between sensory-deprived cortices and nodes of the salience network. In addition, here we show that this is not due to a difference across groups in the organization of the salience network, since we did not find such differences in the relevant ICA component. The driving factors for such reorganization might be the ability and the need for individuals with sensory deprivation to rely on, and identify, external stimuli with the remaining senses, requiring enhanced processing of such stimulus. Since the visual and the auditory cortices are typically not part of the salience network, these connectivity alterations may represent a functional shift (c.f., Cardin et al., 2020 and discussion below, in 4.3), such that sensory regions support the processing of salient stimuli and attention and aid effective switching between networks in the deaf group. Several studies, including the seminal study by MacSweeney et al. (2006), have shown that the superior temporal cortex is important for language processing in the visual domain. While language input in the auditory domain enters the brain within the same lobe, language input from visual language must be conveyed there from the visual region, which might result in weaker functional connectivity within the superior Fig. 3. Regions with stronger connectivity to the seeds from A) the left control network, B) the default network, C) the ventral somatomotor network and D) the attention network for deaf signers, compared to hearing non-signers, in yellow, and in purple for hearing non-signers compared to deaf signers. E) shows an overview of connectivity patterns between seeds and targets. ACC Anterior cingulate cortex; AG Angular gyrus; CU Cuneus; FOG Frontal orbital gyrus; HC Hippocampus; IC Insular cortex; MCC Middle cingulate cortex; MFG Middle frontal gyrus; PCG Pre-/postcentral gyrus; PC Precuneus; SFG Superior frontal gyrus; SMA Supplementary motor area; SMG Supramarginal gyrus; TP Temporal pole. J. Andin and E. Holmer