正在加载图片...
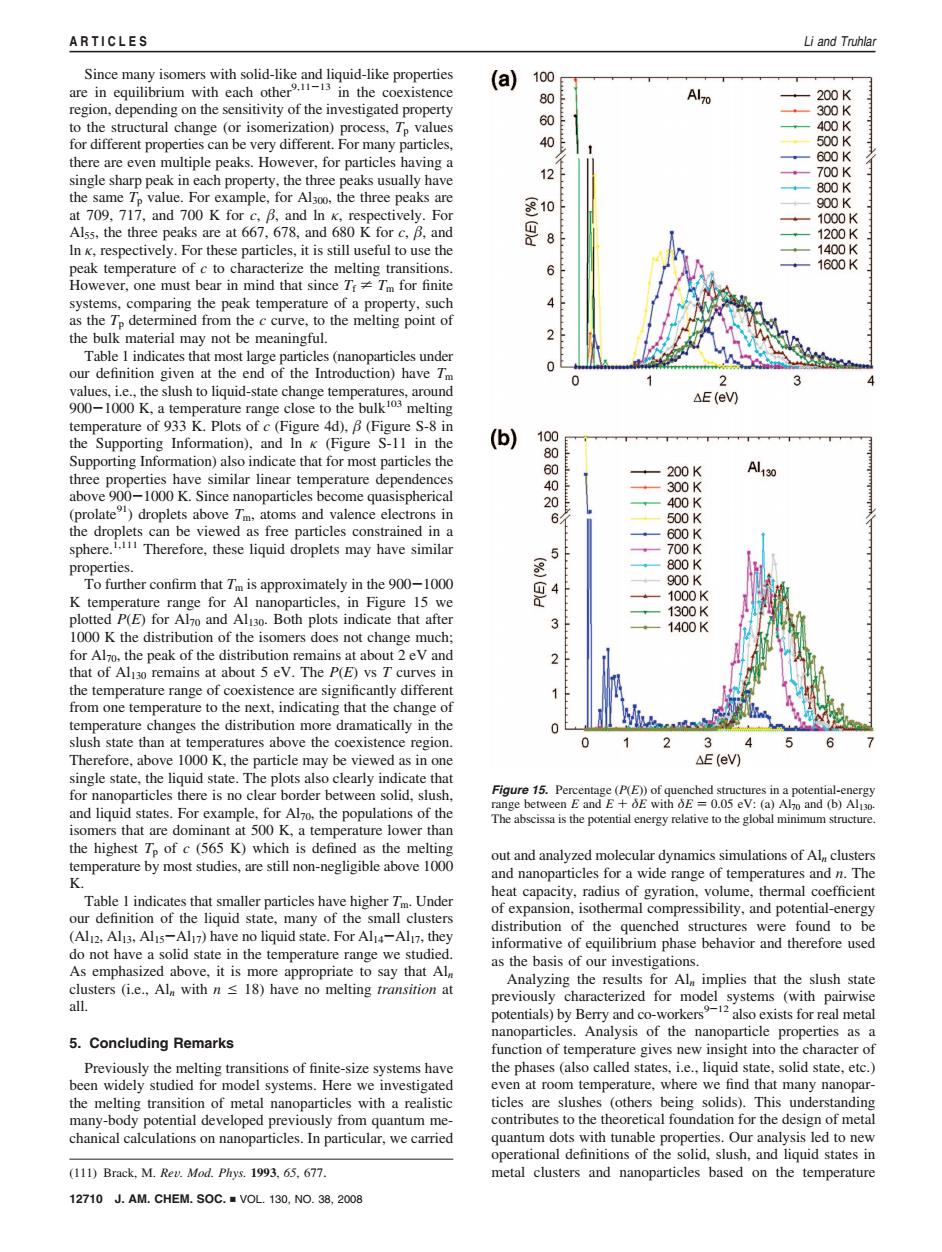
ARTICLES Li and Truhlar Since many isomers with solid-like and liquid-like properties (a) 100 are in equilibrium with each otherin the coexistence 80 Al7o 200K region,depending on the sensitivity of the investigated property 300K to the structural change (or isomerization)process.Tp values 60 400K for different properties can be very different.For many particles, 500K there are even multiple peaks.However,for particles having a 600K single sharp peak in each property,the three peaks usually have 12 700K 800K the same To value.For example,for Al3oo,the three peaks are 900K at 709,717,and 700 K for c.B.and In K,respectively.For 810F 1000K Alss,the three peaks are at 667,678,and 680 K for c,B,and 8 1200K In k,respectively.For these particles,it is still useful to use the 1400K peak temperature of c to characterize the melting transitions. 1600K However,one must bear in mind that since TrTm for finite systems,comparing the peak temperature of a property,such as the Tp determined from the c curve,to the melting point of the bulk material may not be meaningful. Table 1 indicates that most large particles(nanoparticles under our definition given at the end of the Introduction)have Tm values.i.e..the slush to liquid-state change temperatures,around 900-1000 K,a temperature range close to the bulk melting △E(eW) temperature of 933 K.Plots of c(Figure 4d),B(Figure S-8 in the Supporting Information),and In k (Figure S-11 in the (b) 100 Supporting Information)also indicate that for most particles the 80 three properties have similar linear temperature dependences 200K A130 0 300K above 900-1000 K.Since nanoparticles become quasispherical 400K (prolate)droplets above Tm.atoms and valence electrons in 6 500K the droplets can be viewed as free particles constrained in a 600K sphere.Therefore,these liquid droplets may have similar 700K properties. 800K To further confirm that Tm is approximately in the 900-1000 4 900K K temperature range for Al nanoparticles,in Figure 15 we 1000K plotted P(E)for Al7o and Al130.Both plots indicate that after 1300K 3 1400K 1000 K the distribution of the isomers does not change much; for Al7o.the peak of the distribution remains at about 2 eV and that of Ali30 remains at about 5 eV.The P(E)vs T curves in the temperature range of coexistence are significantly different from one temperature to the next,indicating that the change of temperature changes the distribution more dramatically in the slush state than at temperatures above the coexistence region 0 3 4 Therefore,above 1000 K,the particle may be viewed as in one △E(eV single state,the liquid state.The plots also clearly indicate that for nanoparticles there is no clear border between solid,slush. Figure 15.Percentage (P(E))of quenched structures in a potential-energy range between E and E+E with E 0.05 eV:(a)Al7o and (b)Al130 and liquid states.For example,for Al7o.the populations of the The abscissa is the potential energy relative to the global minimum structure. isomers that are dominant at 500 K,a temperature lower than the highest Tp of c(565 K)which is defined as the melting out and analyzed molecular dynamics simulations of Al,clusters temperature by most studies,are still non-negligible above 1000 《 and nanoparticles for a wide range of temperatures and n.The heat capacity,radius of gyration.volume,thermal coefficient Table I indicates that smaller particles have higher Tm.Under of expansion,isothermal compressibility,and potential-energy our definition of the liquid state,many of the small clusters distribution of the quenched structures were found to be (Al12,Al13,Alis-Al7)have no liquid state.For Al14-Al17,they informative of equilibrium phase behavior and therefore used do not have a solid state in the temperature range we studied. as the basis of our investigations As emphasized above,it is more appropriate to say that Al Analyzing the results for Al implies that the slush state clusters (i.e.,Aln with n 18)have no melting transition at previously characterized for model systems (with pairwise all. potentials)by Berry and co-workers-12 also exists for real metal nanoparticles.Analysis of the nanoparticle properties as a 5.Concluding Remarks function of temperature gives new insight into the character of Previously the melting transitions of finite-size systems have the phases (also called states,i.e..liquid state,solid state,etc.) been widely studied for model systems.Here we investigated even at room temperature,where we find that many nanopar- the melting transition of metal nanoparticles with a realistic ticles are slushes (others being solids).This understanding many-body potential developed previously from quantum me- contributes to the theoretical foundation for the design of metal chanical calculations on nanoparticles.In particular,we carried quantum dots with tunable properties.Our analysis led to new operational definitions of the solid,slush,and liquid states in (111)Brack,M.Rev.Mod.Phys.1993,65.677. metal clusters and nanoparticles based on the temperature 12710J.AM.CHEM.S0C.■VOL.130,NO.38,2008Since many isomers with solid-like and liquid-like properties are in equilibrium with each other9,11-13 in the coexistence region, depending on the sensitivity of the investigated property to the structural change (or isomerization) process, Tp values for different properties can be very different. For many particles, there are even multiple peaks. However, for particles having a single sharp peak in each property, the three peaks usually have the same Tp value. For example, for Al300, the three peaks are at 709, 717, and 700 K for c, , and ln κ, respectively. For Al55, the three peaks are at 667, 678, and 680 K for c, , and ln κ, respectively. For these particles, it is still useful to use the peak temperature of c to characterize the melting transitions. However, one must bear in mind that since Tf * Tm for finite systems, comparing the peak temperature of a property, such as the Tp determined from the c curve, to the melting point of the bulk material may not be meaningful. Table 1 indicates that most large particles (nanoparticles under our definition given at the end of the Introduction) have Tm values, i.e., the slush to liquid-state change temperatures, around 900-1000 K, a temperature range close to the bulk103 melting temperature of 933 K. Plots of c (Figure 4d), (Figure S-8 in the Supporting Information), and ln κ (Figure S-11 in the Supporting Information) also indicate that for most particles the three properties have similar linear temperature dependences above 900-1000 K. Since nanoparticles become quasispherical (prolate91) droplets above Tm, atoms and valence electrons in the droplets can be viewed as free particles constrained in a sphere.1,111 Therefore, these liquid droplets may have similar properties. To further confirm that Tm is approximately in the 900-1000 K temperature range for Al nanoparticles, in Figure 15 we plotted P(E) for Al70 and Al130. Both plots indicate that after 1000 K the distribution of the isomers does not change much; for Al70, the peak of the distribution remains at about 2 eV and that of Al130 remains at about 5 eV. The P(E) vs T curves in the temperature range of coexistence are significantly different from one temperature to the next, indicating that the change of temperature changes the distribution more dramatically in the slush state than at temperatures above the coexistence region. Therefore, above 1000 K, the particle may be viewed as in one single state, the liquid state. The plots also clearly indicate that for nanoparticles there is no clear border between solid, slush, and liquid states. For example, for Al70, the populations of the isomers that are dominant at 500 K, a temperature lower than the highest Tp of c (565 K) which is defined as the melting temperature by most studies, are still non-negligible above 1000 K. Table 1 indicates that smaller particles have higher Tm. Under our definition of the liquid state, many of the small clusters (Al12, Al13, Al15-Al17) have no liquid state. For Al14-Al17, they do not have a solid state in the temperature range we studied. As emphasized above, it is more appropriate to say that Aln clusters (i.e., Aln with n e 18) have no melting transition at all. 5. Concluding Remarks Previously the melting transitions of finite-size systems have been widely studied for model systems. Here we investigated the melting transition of metal nanoparticles with a realistic many-body potential developed previously from quantum mechanical calculations on nanoparticles. In particular, we carried out and analyzed molecular dynamics simulations of Aln clusters and nanoparticles for a wide range of temperatures and n. The heat capacity, radius of gyration, volume, thermal coefficient of expansion, isothermal compressibility, and potential-energy distribution of the quenched structures were found to be informative of equilibrium phase behavior and therefore used as the basis of our investigations. Analyzing the results for Aln implies that the slush state previously characterized for model systems (with pairwise potentials) by Berry and co-workers9-12 also exists for real metal nanoparticles. Analysis of the nanoparticle properties as a function of temperature gives new insight into the character of the phases (also called states, i.e., liquid state, solid state, etc.) even at room temperature, where we find that many nanoparticles are slushes (others being solids). This understanding contributes to the theoretical foundation for the design of metal quantum dots with tunable properties. Our analysis led to new operational definitions of the solid, slush, and liquid states in (111) Brack, M. ReV. Mod. Phys. 1993, 65, 677. metal clusters and nanoparticles based on the temperature Figure 15. Percentage (P(E)) of quenched structures in a potential-energy range between E and E + δE with δE ) 0.05 eV: (a) Al70 and (b) Al130. The abscissa is the potential energy relative to the global minimum structure. 12710 J. AM. CHEM. SOC. 9 VOL. 130, NO. 38, 2008 ARTICLES Li and Truhlar